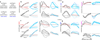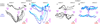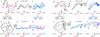Extended Data Figure 10. Urgency and instability in the integration process
a-d, Choice predictive neural activity (top) and psychometric curves (bottom) predicted by several variants of the standard diffusion-to-bound model (see Suppl. information, section 7.7). a, Standard diffusion-to-bound model. Noisy momentary evidence is integrated over time until one of two bounds (+1 or −1; choice 1 or choice 2) is reached. The momentary evidence at each time point is drawn from a Gaussian distribution whose mean corresponds to the coherence of the input, and whose fixed variance is adjusted in each model to achieve the same overall performance (i.e. similar psychometric curves, bottom panels). Coherences are 6, 18, and 50% (the average color coherences in monkey A, Fig. 1b). Average integrated evidence (neural firing rates, arbitrary units) is shown on choice 1 and choice 2 trials (thick vs. thin) for evidence pointing towards choice 1 or choice 2 (solid vs. dashed), on correct trials for all coherences (light gray to black, low to high coherence), and incorrect trials for the lowest coherence (red). The integrated evidence is analogous to the projection of the population response onto the choice axis (e.g. Extended Data Fig. 5b, top-left and bottom-right). b, Urgency model. Here the choice is determined by a race between two diffusion processes (typically corresponding to two hemispheres), one with bound at +1, the other with bound at −1. The diffusion in each process is subject to a constant drift towards the corresponding bound, in addition to the drift provided by the momentary evidence. The input-independent drift implements an ‘urgency’ signal, which guarantees that one of the bounds is reached within a short time. Only the integrated evidence from one of the diffusion processes is shown. The three ‘choice 1’ curves are compressed (in contrast to a) because the urgency signal causes the bound to be reached, and integration toward choice 1 to cease, more quickly than in a. In contrast, the ‘choice 2’ curves are not compressed since the diffusion process that accumulates evidence toward choice 1 never approaches a bound on these trials. c, Same as a, but here the diffusion process is subject to a drift away from the starting point (0) towards the closest bound (+1 or −1). The strength of the drift is proportional to the distance from the starting point, and creates an ‘instability’ at the starting point. d, Same as b, with an instability in the integration as in c for both diffusion processes. The asymmetry between choice 1 and choice 2 curves in b and d resembles the asymmetry in the corresponding PFC curves (Extended Data Figs. 5b,f, upper left). e-j, Neural network model with urgency. This model is based on a similar architecture as the model in Fig. 4. Unlike the neural network in Fig. 4, which was trained solely based on the model output on the last time bin of the trial, here the network is trained based on the output it produces throughout the entire input presentation. The network was trained to reproduce the integrated evidence (i.e. the decision variable) for one of the two diffusion processes (i.e. one of the two ‘hemispheres’) in a diffusion-to-bound model with urgency (b, see Suppl. Information, section 7.7). Similar conventions as in Fig. 5. The urgency signal is controlled by an additional binary input into the network. Here, the urgency and sensory inputs are turned off as soon as a bound is reached. The network generates only a single, stable fixed point in each context, corresponding to the decision boundary (large red cross). The model also implements a series of points of relatively slow dynamics (small red crosses) approximately lying on a single curve. The axes of slow dynamics at these slow points (red lines) are locally aligned. Notably, responses at these slow points have a strong tendency to drift towards the single, stable fixed point (the decision boundary), and thus the curve of slow points does not correspond to an approximate line attractor. This drift implements the urgency signal and causes an asymmetry in the trajectories, which converge on a single point for choice 1, but have endpoints that are parametrically ordered by coherence along the choice axis for choice 2. As discussed below (panel r), this model relies on the same mechanism of selection as the original model (Fig. 5, see main text). k-p, Neural network model with instability. Trajectories show simulated population responses for a model (same architecture as in Fig. 4) that was trained to solve the context-dependent task (Fig. 1) only on high-coherence stimuli and in the absence of internal noise (see Suppl. Information, section 7.7). Same conventions as in Fig. 5. In the absence of noise, prolonged integration of evidence is not necessary for accurate performance on the task. As a consequence, the model implements a saddle point (blue cross) instead of an approximate line attractor. Points of slow dynamics (small red crosses, obscured by the red lines) occur only close to the saddle point. The right-zero-eigenvectors of the linearized dynamics around these slow points (red lines) correspond to the directions of slowest dynamics, and determine the direction of the axis of choice. When displaced from the saddle point, the responses quickly drift towards one of the two stable attractors (large red crosses) corresponding to the choices. For a given choice, trajectories for all coherences therefore end in the same location along the choice axis, in contrast to the responses in the original model (Fig. 5). Despite these differences, the original model (Fig. 5) and the network model with instability (k-p) rely on a common mechanism of context-dependent selection (see panel s). q-s, Dynamical features (key, bottom) underlying input selection and choice in three related neural network models. All models are based on a common architecture (Fig. 4) but are the result of different training procedures. q, Dynamical features of the model described in the main paper (Figs. 5–6), re-plotted from Fig. 6c. r, The urgency model (e-j). s, The instability model (k-p). In all models, the developing choice is implemented as more or less gradual movement along an axis of slow dynamics (specified by the locally computed right-eigenvectors associated with the near-zero eigenvalue of the linearized dynamics, red lines). The inputs are selected, i.e. result in movement along the axis of slow dynamics, depending on their projection onto the selection vector (the locally computed left-eigenvectors associated with the near-zero eigenvalue). In this sense, the three models implement the same mechanisms of context-dependent selection and choice.