The ATP-binding cassette transporter-2 (ABCA2) regulates esterification of plasma membrane cholesterol by modulation of sphingolipid metabolism
- PMID: 24201375
- PMCID: PMC4437569
- DOI: 10.1016/j.bbalip.2013.10.019
The ATP-binding cassette transporter-2 (ABCA2) regulates esterification of plasma membrane cholesterol by modulation of sphingolipid metabolism
Abstract
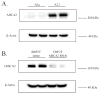
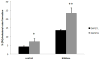
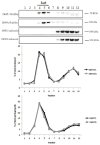
The ATP-binding cassette transporters are a large family (~48 genes divided into seven families A-G) of proteins that utilize the energy of ATP-hydrolysis to pump substrates across lipid bilayers against a concentration gradient. The ABC "A" subfamily is comprised of 13 members and transport sterols, phospholipids and bile acids. ABCA2 is the most abundant ABC transporter in human and rodent brain with highest expression in oligodendrocytes, although it is also expressed in neurons. Several groups have studied a possible connection between ABCA2 and Alzheimer's disease as well as early atherosclerosis. ABCA2 expression levels have been associated with changes in cholesterol and sphingolipid metabolism. In this paper, we hypothesized that ABCA2 expression level may regulate esterification of plasma membrane-derived cholesterol by modulation of sphingolipid metabolism. ABCA2 overexpression in N2a neuroblastoma cells was associated with an altered bilayer distribution of the sphingolipid ceramide that inhibited acylCoA:cholesterol acyltransferase (ACAT) activity and cholesterol esterification. In contrast, depletion of endogenous ABCA2 in the rat schwannoma cell line D6P2T increased esterification of plasma membrane cholesterol following treatment with exogenous bacterial sphingomyelinase. These findings suggest that control of ABCA2 expression level may be a key locus of regulation for esterification of plasma membrane-derived cholesterol through modulation of sphingolipid metabolism.
Keywords: ABCA2; Ceramide; Cholesterol; Esterification; Sphingomyelin; Transporter.
© 2013.
Figures







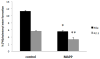


Similar articles
-
The ATP-Binding Cassette Transporter-2 (ABCA2) Overexpression Modulates Sphingosine Levels and Transcription of the Amyloid Precursor Protein (APP) Gene.Curr Alzheimer Res. 2015;12(9):847-59. doi: 10.2174/156720501209151019105834. Curr Alzheimer Res. 2015. PMID: 26510981 Free PMC article.
-
The ATP-binding cassette transporter-2 (ABCA2) regulates cholesterol homeostasis and low-density lipoprotein receptor metabolism in N2a neuroblastoma cells.Biochim Biophys Acta. 2011 Dec;1811(12):1152-64. doi: 10.1016/j.bbalip.2011.07.010. Epub 2011 Jul 23. Biochim Biophys Acta. 2011. PMID: 21810484 Free PMC article.
-
Human ATP-binding cassette transporter-2 (ABCA2) positively regulates low-density lipoprotein receptor expression and negatively regulates cholesterol esterification in Chinese hamster ovary cells.Biochim Biophys Acta. 2004 Jul 5;1683(1-3):89-100. doi: 10.1016/j.bbalip.2004.04.009. Biochim Biophys Acta. 2004. PMID: 15238223
-
Sphingolipid synthetic pathways are major regulators of lipid homeostasis.Adv Exp Med Biol. 2011;721:139-48. doi: 10.1007/978-1-4614-0650-1_9. Adv Exp Med Biol. 2011. PMID: 21910087 Review.
-
ATP-binding cassette transporter-2 (ABCA2) as a therapeutic target.Biochem Pharmacol. 2018 May;151:188-200. doi: 10.1016/j.bcp.2017.11.018. Epub 2017 Dec 6. Biochem Pharmacol. 2018. PMID: 29223352 Free PMC article. Review.
Cited by
-
Emerging Role of ABC Transporters in Glia Cells in Health and Diseases of the Central Nervous System.Cells. 2024 Apr 24;13(9):740. doi: 10.3390/cells13090740. Cells. 2024. PMID: 38727275 Free PMC article. Review.
-
Strategies to gain novel Alzheimer's disease diagnostics and therapeutics using modulators of ABCA transporters.Free Neuropathol. 2021;2:33. doi: 10.17879/freeneuropathology-2021-3528. Epub 2021 Dec 13. Free Neuropathol. 2021. PMID: 34977908 Free PMC article.
-
The ATP-Binding Cassette Transporter-2 (ABCA2) Overexpression Modulates Sphingosine Levels and Transcription of the Amyloid Precursor Protein (APP) Gene.Curr Alzheimer Res. 2015;12(9):847-59. doi: 10.2174/156720501209151019105834. Curr Alzheimer Res. 2015. PMID: 26510981 Free PMC article.
-
Increased Expression of the ABCA1 and ABCA3 Transporter Genes is Associated with Cisplatin Resistance in Breast Cancer Cells.Asian Pac J Cancer Prev. 2023 Nov 1;24(11):3969-3977. doi: 10.31557/APJCP.2023.24.11.3969. Asian Pac J Cancer Prev. 2023. PMID: 38019257 Free PMC article.
-
Quantitative Systems Pharmacology Model for Alzheimer Disease Indicates Targeting Sphingolipid Dysregulation as Potential Treatment Option.CPT Pharmacometrics Syst Pharmacol. 2018 Nov;7(11):759-770. doi: 10.1002/psp4.12351. Epub 2018 Oct 8. CPT Pharmacometrics Syst Pharmacol. 2018. PMID: 30207429 Free PMC article.
References
-
- Voloshyna I, Reiss AB. The ABC transporters in lipid flux and atherosclerosis. Prog Lipid Res. 2011;50:213–224. - PubMed
-
- Higgins CF, Linton KJ. The ATP switch model for ABC transporters. Nat Struct Mol Biol. 2004;11:918–926. - PubMed
-
- Takahashi K, Kimura Y, Nagata K, Yamamoto A, Matsuo M, Ueda K. ABC proteins: key molecules for lipid homeostasis. Med Mol Morphol. 2005;38:2–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

