Pancreatic stellate cells promote hapto-migration of cancer cells through collagen I-mediated signalling pathway
- PMID: 24201748
- PMCID: PMC3899756
- DOI: 10.1038/bjc.2013.706
Pancreatic stellate cells promote hapto-migration of cancer cells through collagen I-mediated signalling pathway
Abstract
Background: Pancreatic stellate cells (PSCs) promote metastasis as well as local growth of pancreatic cancer. However, the factors mediating the effect of PSCs on pancreatic cancer cells have not been clearly identified.
Methods: We used a modified Boyden chamber assay as an in vitro model to investigate the role of PSCs in migration of Panc1 and UlaPaCa cells and to identify the underlying mechanisms.
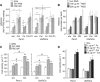
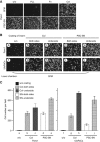
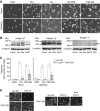
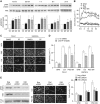
Results: PSC supernatant (PSC-SN) dose-dependently induced the trans-migration of Panc1 and UlaPaCa cells, mainly via haptokinesis and haptotaxis, respectively. In contrast to poly-L-lysine or fibronectin, collagen I resembled PSC-SN with respect to its effect on cancer cell behaviours, including polarised morphology, facilitated adhesion, accelerated motility and stimulated trans-migration. Blocking antibodies against integrin α2/β1 subunits significantly attenuated PSC-SN- or collagen I-promoted cell trans-migration and adhesion. Moreover, both PSC-SN and collagen I induced the formation of F-actin and focal adhesions in cells, which was consistent with the constantly enhanced phosphorylation of focal adhesion kinase (FAK, Tyr397). Inhibition of FAK function by an inhibitor or small interference RNAs significantly diminished the effect of PSC-SN or collagen I on haptotaxis/haptokinesis of pancreatic cancer cells.
Conclusion: Collagen I is the major mediator for PSC-SN-induced haptokinesis of Panc1 and haptotaxis of UlaPaCa by activating FAK signalling via binding to integrin α2β1.
Figures



References
-
- Apte MV, Park S, Phillips PA, Santucci N, Goldstein D, Kumar RK, Ramm GA, Buchler M, Friess H, McCarroll JA, Keogh G, Merrett N, Pirola R, Wilson JS. Desmoplastic reaction in pancreatic cancer: role of pancreatic stellate cells. Pancreas. 2004;29:179–187. - PubMed
-
- Arao S, Masumoto A, Otsuki M. Beta1 integrins play an essential role in adhesion and invasion of pancreatic carcinoma cells. Pancreas. 2000;20:129–137. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

