Aven is dynamically regulated during Xenopus oocyte maturation and is required for oocyte survival
- PMID: 24201807
- PMCID: PMC3847313
- DOI: 10.1038/cddis.2013.435
Aven is dynamically regulated during Xenopus oocyte maturation and is required for oocyte survival
Abstract
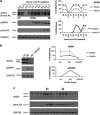
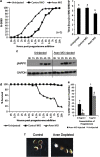
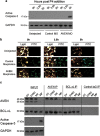
We have analyzed the expression and function of the cell death and cell cycle regulator Aven in Xenopus. Analysis of Xenopus Aven expression in oocytes and embryos revealed a band close to the predicted molecular weight of the protein (36 kDa) in addition to two bands of higher molecular weight (46 and 49 kDa), one of which was determined to be due to phosphorylation of the protein. The protein is primarily detected in the cytoplasm of oocytes and is tightly regulated during meiotic and mitotic cell cycles. Progesterone stimulation of oocytes resulted in a rapid loss of Aven expression with the protein levels recovering before germinal vesicle breakdown (GVBD). This loss of Aven is required for the G2-M1 cell cycle transition. Aven morpholino knockdown experiments revealed that early depletion of the protein increases progesterone sensitivity and facilitates GVBD, but prolonged depletion of Aven results in caspase-3 activation and oocyte death by apoptosis. Phosphorylated Aven (46 kDa) was found to bind Bcl-xL in oocytes, but this interaction was lost in apoptotic oocytes. Thus, Aven alters progesterone sensitivity in oocytes and is critical for oocyte survival.
Figures

References
-
- Maller JL, Krebs EG. Progesterone-stimulated meiotic cell division in Xenopus oocytes. Induction by regulatory subunit and inhibition by catalytic subunit of adenosine 3':5'-monophosphate-dependent protein kinase. J Biol Chem. 1977;252:1712–1718. - PubMed
-
- Finidori-Lepicard J, Schorderet-Slatkine S, Hanoune J, Baulieu EE. Progesterone inhibits membrane-bound adenylate cyclase in Xenopus laevis oocytes. Nature. 1981;292:255–257. - PubMed
-
- Sadler SE, Maller JL. Progesterone inhibits adenylate cyclase in Xenopus oocytes. Action on the guanine nucleotide regulatory protein. J Biol Chem. 1981;256:6368–6373. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

