Sildenafil exposure and hemodynamic effect after Fontan surgery
- PMID: 24201857
- PMCID: PMC3887448
- DOI: 10.1097/PCC.0000000000000007
Sildenafil exposure and hemodynamic effect after Fontan surgery
Abstract
Objective: Determine sildenafil exposure and hemodynamic effect in children after Fontan single-ventricle surgery.
Design: Prospective dose-escalation trial.
Setting: Single-center pediatric catheterization laboratory.
Patients: Nine children post Fontan single-ventricle surgical palliation and undergoing elective cardiac catheterization: median (range) age and weight, 5.2 years (2.5-9.4 yr) and 16.3 kg (9.5-28.1 kg). Five children (55%) were boys, and six of nine (67%) had a systemic right ventricle.
Interventions: Catheterization and echocardiography performed before and immediately after single-dose IV sildenafil (0.25, 0.35, or 0.45 mg/kg over 20 min).
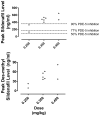
Measurements and main results: Peak sildenafil and desmethyl sildenafil concentration, change in hemodynamic variables measured by cardiac catheterization and echocardiography. Maximum sildenafil concentrations ranged from 124 to 646 ng/mL and were above the in vitro threshold needed for 77% phosphodiesterase type-5 inhibition in eight of nine children and 90% inhibition in seven of seven children with doses more than or equal to 0.35 mg/kg. Sildenafil improved stroke volume (+22%, p = 0.05) and cardiac output (+10%, p = 0.01) with no significant change in heart rate in eight of nine children. Sildenafil also lowered systemic (-16%, p = 0.01) and pulmonary vascular resistance index in all nine children (median baseline pulmonary vascular resistance index 2.4 [range, 1.3-3.7]; decreased to 1.9 [0.8-2.7] Wood Units × m; p = 0.01) with no dose-response effect. Pulmonary arterial pressures decreased (-10%, p = 0.02) and pulmonary blood flow increased (9%, p = 0.02). There was no change in myocardial performance index and no adverse events.
Conclusions: After Fontan surgery, sildenafil infusion acutely improves cardiopulmonary hemodynamics, increasing cardiac index. For the range of doses studied, exposure was within the acute safety range reported in adult subjects.
Trial registration: ClinicalTrials.gov NCT01169519.
Figures


References
-
- O’Leary PW. Prevalence, clinical presentation and natural history of patients with single ventricle. Prog Pediatr Cardiol. 2002;16:31–8.
-
- Gentles TL, Gauvreau K, Mayer JE, Jr, et al. Functional outcome after the Fontan operation: factors influencing late morbidity. J Thorac Cardiovasc Surg. 1997;114:392–403. discussion 4–5. - PubMed
-
- Gersony WM. Fontan operation after 3 decades: what we have learned. Circulation. 2008;117:13–5. - PubMed
-
- Fixler DE, Nembhard WN, Salemi JL, Ethen MK, Canfield MA. Mortality in first 5 years in infants with functional single ventricle born in Texas, 1996 to 2003. Circulation. 2010;121:644–50. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- HHSN267200700051C/HD/NICHD NIH HHS/United States
- K24 HD058735/HD/NICHD NIH HHS/United States
- RC1 HL099941/HL/NHLBI NIH HHS/United States
- 1K23HD064814/HD/NICHD NIH HHS/United States
- K23 HD068497/HD/NICHD NIH HHS/United States
- R01 HD057956/HD/NICHD NIH HHS/United States
- HHSN275201000003C/HD/NICHD NIH HHS/United States
- K23 HD064814/HD/NICHD NIH HHS/United States
- HHSN275201000003I/HD/NICHD NIH HHS/United States
- HHSN267200700051C/DK/NIDDK NIH HHS/United States
- 1K24HD058735-05/HD/NICHD NIH HHS/United States
- 1R01HD057956-05/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

