Safety and on-treatment efficacy of telaprevir: the early access programme for patients with advanced hepatitis C
- PMID: 24201995
- PMCID: PMC4078754
- DOI: 10.1136/gutjnl-2013-305667
Safety and on-treatment efficacy of telaprevir: the early access programme for patients with advanced hepatitis C
Abstract
Background and aim: Severe adverse events (AEs) compromise the outcome of direct antiviral agent-based treatment in patients with advanced liver fibrosis due to HCV infection. HEP3002 is an ongoing multinational programme to evaluate safety and efficacy of telaprevir (TVR) plus pegylated-interferon-α (PEG-IFNα) and ribavirin (RBV) in patients with advanced liver fibrosis caused by HCV genotype 1 (HCV-1).
Methods: 1782 patients with HCV-1 and bridging fibrosis or compensated cirrhosis were prospectively recruited from 16 countries worldwide, and treated with 12 weeks of TVR plus PEG-IFN/RBV, followed by 12 or 36 weeks of PEG-IFN and RBV (PR) alone dependent on virological response to treatment and previous response type.
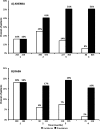
Results: 1587 patients completed 12 weeks of triple therapy and 4 weeks of PR tail (53% cirrhosis, 22% HCV-1a). By week 12, HCV RNA was undetectable in 85% of naives, 88% of relapsers, 80% of partial responders and 72% of null responders. Overall, 931 patients (59%) developed grade 1-4 anaemia (grade 3/4 in 31%), 630 (40%) dose reduced RBV, 332 (21%) received erythropoietin and 157 (10%) were transfused. Age and female gender were the strongest predictors of anaemia. 64 patients (4%) developed a grade 3/4 rash. Discontinuation of TVR due to AEs was necessary in 193 patients (12%). Seven patients died (0.4%, six had cirrhosis).
Conclusions: In compensated patients with advanced fibrosis due to HCV-1, triple therapy with TVR led to satisfactory rates of safety, tolerability and on-treatment virological response with adequate managements of AEs.
Keywords: ANEMIA; HEPATITIS C; INTERFERON.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures

Comment in
-
Beyond phase 3 registration trials: defining safety for triple therapy with protease inhibitors in cirrhosis.Gut. 2014 Jul;63(7):1033-4. doi: 10.1136/gutjnl-2013-306480. Epub 2013 Dec 13. Gut. 2014. PMID: 24334256 No abstract available.
References
-
- European Association for the Study of the Liver (EASL). EASL clinical practice guidelines: management of hepatitis C virus infection. J Hepatol 2011;55:245–64 - PubMed
-
- Dusheiko G, Wedemeyer H. New Protease inhibitors and direct-acting antivirals for hepatitis C: interferon's long goodbye. Gut 2012;61:1647–52 - PubMed
-
- Sarrazin C, Hezode C, Zeuzem S, et al. Antiviral strategies in hepatitis C virus infection. J Hepatol 2012;56(Suppl 1):S88–100 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
