Lack of positive effect of intravitreal bevacizumab in central serous chorioretinopathy: meta-analysis and review
- PMID: 24202051
- PMCID: PMC3869506
- DOI: 10.1038/eye.2013.236
Lack of positive effect of intravitreal bevacizumab in central serous chorioretinopathy: meta-analysis and review
Abstract
Purpose: To review and evaluate the effects of intravitreal bevacizumab injection (IVB) in centralserous chorioretinopathy (CSC) by meta-analysis.
Patients and methods: Clinical controlled studies that evaluated the effect of IVB in CSC were identified through systematic searches of Embase, PubMed, and the Cochrane Central Register of Controlled Trials. Data on the best-corrected visual acuity (BCVA) in logMAR and central macular thickness (CMT) in μm at baseline and 6 months after IVB were extracted and compared with those treated by simple observation.
Results: Four clinical controlled studies were included in the meta-analysis. The IVB injection group achieved better BCVA at a follow-up of 6 months. However, the analysis showed that there were no significant differences of BCVA at 6 months after injection between IVB group and the observation group (-0.02 logMAR, 95% CI -0.14 to 0.11, P=0.80). The analysis of the reduction in CMT revealed that the difference between groups was not statistically significant (-8.37 μm, 95% CI -97.26 to 80.52, P=0.85). No report assessed severe complications or side effects of IVB in patients with CSC.
Conclusions: Meta-analysis failed to verify the positive effect of IVB in CSC based on the epidemiological literature published to date.
Figures
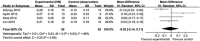
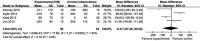
Comment in
-
Reply to 'Lack of positive effect of intravitreal bevacizumab in central serous chorioretinopathy: meta-analysis and review'.Eye (Lond). 2015 Jul;29(7):978. doi: 10.1038/eye.2015.5. Epub 2015 Feb 27. Eye (Lond). 2015. PMID: 25721515 Free PMC article. No abstract available.
-
Methodological remarks concerning the recent meta-analysis on the effect of intravitral bevacizumab in central serous chorioretinopathy.Eye (Lond). 2015 Jul;29(7):978. doi: 10.1038/eye.2015.8. Epub 2015 Feb 27. Eye (Lond). 2015. PMID: 25721516 Free PMC article. No abstract available.
References
-
- Seong HK, Bae JH, Kim ES, Han JR, Nam WH, Kim HK. Intravitreal bevacizumab to treat acute central serous chorioretinopathy: short-term effect. Ophthalmologica. 2009;223 (5:343–347. - PubMed
-
- Torres-Soriano ME, Garcia-Aguirre G, Kon-Jara V, Ustariz-Gonzales O, Abraham-Marin M, Ober MD, et al. A pilot study of intravitreal bevacizumab for the treatment of central serous chorioretinopathy (case reports) Graefes Arch Clin Exp Ophthalmol. 2008;246 (9:1235–1239. - PubMed
-
- Li XJ, Zhang JS. Intravitreal bevacizumab injection for chronic central serous chorioretinopathy. Chin Med J (Engl) 2010;123 (15:2145–2147. - PubMed
-
- Yannuzzi LA. Type-A behavior and central serous chorioretinopathy. Retina. 1987;7 (2:111–131. - PubMed
-
- Lafaut BA, Salati C, Priem H, De Laey JJ. Indocyanine green angiography is of value for the diagnosis of chronic central serous chorioretinopathy in elderly patients. Graefes Arch Clin Exp Ophthalmol. 1998;236 (7:513–521. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

