Activation of the mitochondrial caspase pathway and subsequent calpain activation in monkey RPE cells cultured under zinc depletion
- PMID: 24202052
- PMCID: PMC3890765
- DOI: 10.1038/eye.2013.239
Activation of the mitochondrial caspase pathway and subsequent calpain activation in monkey RPE cells cultured under zinc depletion
Abstract
Purpose: Decreased zinc levels in the macula are reported in patients with age-related macular degeneration, and the zinc chelator N,N,N',N'-tetrakis (2- pyridylmethyl) ethylenediamine) (TPEN) causes death of human retinal pigment epithelial (RPE) cells. The purpose of the present study was to investigate signal transduction pathways during cell death initiated by TPEN, using monkey RPE cells.
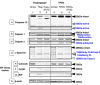
Methods: RPE cells were cultured with TPEN. Activation of calpains and caspases, and proteolysis of their substrates were detected by immunoblotting. Incubation of calpain inhibitor SNJ-1945 or caspase inhibitor z-VAD-fmk was used to confirm activation of specific proteases.
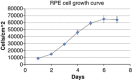
Results: TPEN caused a time-dependent decrease in viable RPE cells. Cell death was accompanied by activation of calpain-1, caspase-9, and caspase-3. SNJ-1945 inhibited calpain activation and slightly inhibited caspase-9 activation. z-VAD-fmk inhibited caspases and calpain-1 activation. TPEN did not activate caspase-12.
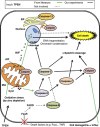
Conclusions: Relative zinc deficiency in RPE cells causes activation of cytosolic calpain and mitochondrial caspase pathways without ER stress.
Figures


Similar articles
-
Contribution of Calpain and Caspases to Cell Death in Cultured Monkey RPE Cells.Invest Ophthalmol Vis Sci. 2017 Oct 1;58(12):5412-5420. doi: 10.1167/iovs.17-22325. Invest Ophthalmol Vis Sci. 2017. PMID: 29053764 Free PMC article.
-
Contribution of calpain to cellular damage in human retinal pigment epithelial cells cultured with zinc chelator.Curr Eye Res. 2007 Jun;32(6):565-73. doi: 10.1080/02713680701359633. Curr Eye Res. 2007. PMID: 17612972
-
Depletion of intracellular zinc and copper with TPEN results in apoptosis of cultured human retinal pigment epithelial cells.Invest Ophthalmol Vis Sci. 2001 Feb;42(2):460-5. Invest Ophthalmol Vis Sci. 2001. PMID: 11157883
-
Mechanisms of apoptosis in human retinal pigment epithelium induced by TNF-alpha in conditions of heavy metal ion deficiency.Invest Ophthalmol Vis Sci. 2005 Mar;46(3):1039-46. doi: 10.1167/iovs.04-0325. Invest Ophthalmol Vis Sci. 2005. PMID: 15728563
-
Caspase Activation and Inhibition.Cold Spring Harb Perspect Biol. 2022 Aug 1;14(8):a041020. doi: 10.1101/cshperspect.a041020. Cold Spring Harb Perspect Biol. 2022. PMID: 35914782 Free PMC article. Review. No abstract available.
Cited by
-
Zinc at the crossroads of exercise and proteostasis.Redox Biol. 2020 Aug;35:101529. doi: 10.1016/j.redox.2020.101529. Epub 2020 Apr 1. Redox Biol. 2020. PMID: 32273258 Free PMC article. Review.
-
Medication Trends for Age-Related Macular Degeneration.Int J Mol Sci. 2021 Oct 31;22(21):11837. doi: 10.3390/ijms222111837. Int J Mol Sci. 2021. PMID: 34769270 Free PMC article. Review.
-
Activation of Cytosolic Calpain, Not Caspase, Is Underlying Mechanism for Hypoxic RGC Damage in Human Retinal Explants.Invest Ophthalmol Vis Sci. 2020 Nov 2;61(13):13. doi: 10.1167/iovs.61.13.13. Invest Ophthalmol Vis Sci. 2020. PMID: 33156340 Free PMC article.
-
Calpains as mechanistic drivers and therapeutic targets for ocular disease.Trends Mol Med. 2022 Aug;28(8):644-661. doi: 10.1016/j.molmed.2022.05.007. Epub 2022 May 29. Trends Mol Med. 2022. PMID: 35641420 Free PMC article. Review.
-
Potential effect of luteolin, epiafzelechin, and albigenin on rats under cadmium-induced inflammatory insult: In silico and in vivo approach.Front Chem. 2023 Mar 1;11:1036478. doi: 10.3389/fchem.2023.1036478. eCollection 2023. Front Chem. 2023. PMID: 36936530 Free PMC article.
References
-
- Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005;85:845–881. - PubMed
-
- Piguet B, Haimovici R, Bird AC. Dominantly inherited drusen represent more than one disorder: a historical review. Eye (Lond) 1995;9 (Pt 1:34–41. - PubMed
-
- Newsome DA, Miceli MV, Tate DJ, Jr, Alcock NW, Oliver PD. Zinc content of human retinal pigment epithelium decreases with age and macular degeneration, but superoxide dismutase activity increases. J Trace Elem Exp Med. 1995;8:193–199.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

