Identification of a novel flavonoid glycoside sulfotransferase in Arabidopsis thaliana
- PMID: 24202284
- PMCID: PMC3905610
- DOI: 10.1093/jb/mvt102
Identification of a novel flavonoid glycoside sulfotransferase in Arabidopsis thaliana
Abstract
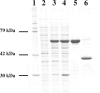
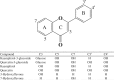
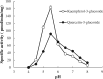
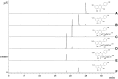
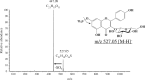
The discovery of sulfated flavonoids in plants suggests that sulfation may play a regulatory role in the physiological functions of flavonoids. Sulfation of flavonoids is mediated by cytosolic sulfotransferases (SULTs), which utilize 3'-phosphoadenosine 5'-phosphosulfate (PAPS) as the sulfate donor. A novel SULT from Arabidopsis thaliana, designated AtSULT202B7 (AGI code: At1g13420), was cloned and expressed in Escherichia coli. Using various compounds as potential substrates, we demonstrated, for the first time, that AtSULT202B7 displayed sulfating activity specific for flavonoids. Intriguingly, the recombinant enzyme preferred flavonoid glycosides (e.g. kaempferol-3-glucoside and quercetin-3-glucoside) rather than their aglycone counterparts. Among a series of hydroxyflavones tested, AtSULT202B7 showed the enzymatic activity only for 7-hydroxyflavone. pH-dependency study showed that the optimum pH was relatively low (pH 5.5) compared with those (pH 6.0-8.5) previously reported for other isoforms. Based on the comparison of high performance (pressure) liquid chromatography (HPLC) retention times between sulfated kaempferol and the deglycosylated product of sulfated kaempferol-3-glucoside, the sulfation site in sulfated kaempferol-3-glucoside appeared to be the hydroxyl group of the flavonoid skeleton. In addition, by using direct infusion mass spectrometry, it was found that the sulfated product had one sulfonate group within the molecule. These results indicated that AtSULT202B7 functions as a flavonoid glycoside 7-sulfotransferase.
Keywords: Arabidopsis thaliana; AtSULT202B7; flavonoid glycoside; sulfation; sulfotransferase.
Figures

References
-
- Mol J, Grotewoldb E, Koesa R. How genes paint flowers and seeds. Trends Plant Sci. 1998;3:212–217.
-
- Treutter D. Significance of flavonoids in plant resistance and enhancement of their biosynthesis. Plant Biol. (Stuttg) 2005;7:581–591. - PubMed
-
- Hassan S, Mathesius U. The role of flavonoids in root-rhizosphere signalling: opportunities and challenges for improving plant-microbe interactions. J. Exp. Bot. 2012;63:3429–3444. - PubMed
-
- Peer WA, Murphy AS. Flavonoids and auxin transport: modulators or regulators? Trends Plant Sci. 2007;12:556–563. - PubMed
-
- Williams CA, Grayer RJ. Anthocyanins and other flavonoids. Nat. Prod. Rep. 2004;21:539–573. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

