Role of Hydroxamate-Based Histone Deacetylase Inhibitors (Hb-HDACIs) in the Treatment of Solid Malignancies
- PMID: 24202327
- PMCID: PMC3795372
- DOI: 10.3390/cancers5030919
Role of Hydroxamate-Based Histone Deacetylase Inhibitors (Hb-HDACIs) in the Treatment of Solid Malignancies
Abstract
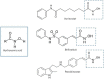
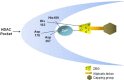
Hydroxamate-based histone deacetylase inhibitors (Hb-HDACIs), such as vorinostat, belinostat and panobinostat, have been previously shown to have a wide range of activity in hematologic malignancies such as cutaneous T-cell lymphoma and multiple myeloma. Recent data show that they synergize with a variety of cytotoxic and molecular targeted agents in many different solid tumors, including breast, prostate, pancreatic, lung and ovarian cancer. Hb-HDACIs have a quite good toxicity profile and are now being tested in phase I and II clinical trials in solid tumors with promising results in selected neoplasms, such as hepatocarcinoma. This review will focus on their clinical activity and safety in patients with advanced solid neoplasms.
Figures
Similar articles
-
Subchronic Toxicities of HZ1006, a Hydroxamate-Based Histone Deacetylase Inhibitor, in Beagle Dogs and Sprague-Dawley Rats.Int J Environ Res Public Health. 2016 Nov 30;13(12):1190. doi: 10.3390/ijerph13121190. Int J Environ Res Public Health. 2016. PMID: 27916918 Free PMC article.
-
Recent Progress in Histone Deacetylase Inhibitors as Anticancer Agents.Curr Med Chem. 2020;27(15):2449-2493. doi: 10.2174/0929867325666181016163110. Curr Med Chem. 2020. PMID: 30332940 Review.
-
An overview of investigational Histone deacetylase inhibitors (HDACis) for the treatment of non-Hodgkin's lymphoma.Expert Opin Investig Drugs. 2016 Jun;25(6):687-96. doi: 10.1517/13543784.2016.1164140. Epub 2016 Mar 25. Expert Opin Investig Drugs. 2016. PMID: 26954526 Review.
-
Histone deacetylase inhibitors as a new anticancer option: How far can we go with expectations? delivery systems.J BUON. 2018 Jul-Aug;23(4):846-861. J BUON. 2018. PMID: 30358185
-
Histone deacetylase inhibitors and pancreatic cancer: are there any promising clinical trials?World J Gastroenterol. 2013 Feb 28;19(8):1173-81. doi: 10.3748/wjg.v19.i8.1173. World J Gastroenterol. 2013. PMID: 23482354 Free PMC article. Review.
Cited by
-
Molecular Research in Pancreatic Cancer: Small Molecule Inhibitors, Their Mechanistic Pathways and Beyond.Curr Issues Mol Biol. 2023 Feb 27;45(3):1914-1949. doi: 10.3390/cimb45030124. Curr Issues Mol Biol. 2023. PMID: 36975494 Free PMC article. Review.
-
Molecular therapy for acute myeloid leukaemia.Nat Rev Clin Oncol. 2016 May;13(5):305-18. doi: 10.1038/nrclinonc.2015.210. Epub 2015 Dec 1. Nat Rev Clin Oncol. 2016. PMID: 26620272 Free PMC article. Review.
-
Loss of the deubiquitylase BAP1 alters class I histone deacetylase expression and sensitivity of mesothelioma cells to HDAC inhibitors.Oncotarget. 2015 May 30;6(15):13757-71. doi: 10.18632/oncotarget.3765. Oncotarget. 2015. PMID: 25970771 Free PMC article.
-
Methods for Hydroxamic Acid Synthesis.Curr Org Chem. 2019;23(9):978-993. doi: 10.2174/1385272823666190424142821. Curr Org Chem. 2019. PMID: 32565717 Free PMC article.
-
New insights on the role of epigenetic alterations in hepatocellular carcinoma.J Hepatocell Carcinoma. 2014 Jun 12;1:65-83. doi: 10.2147/JHC.S44506. eCollection 2014. J Hepatocell Carcinoma. 2014. PMID: 27508177 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

