Fas (CD95) expression in myeloid cells promotes obesity‐induced muscle insulin resistance
- PMID: 24203314
- PMCID: PMC3936487
- DOI: 10.1002/emmm.201302962
Fas (CD95) expression in myeloid cells promotes obesity‐induced muscle insulin resistance
Abstract
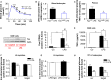
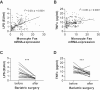
Low-grade inflammation in adipose tissue and liver has been implicated in obesity-associated insulin resistance and type 2 diabetes. Yet, the contribution of inflammatory cells to the pathogenesis of skeletal muscle insulin resistance remains elusive. In a large cohort of obese human individuals, blood monocyte Fas (CD95) expression correlated with systemic and skeletal muscle insulin resistance. To test a causal role for myeloid cell Fas expression in the development of skeletal muscle insulin resistance, we generated myeloid/haematopoietic cell-specific Fas-depleted mice. Myeloid/haematopoietic Fas deficiency prevented the development of glucose intolerance in high fat-fed mice, in ob/ob mice, and in mice acutely challenged by LPS. In vivo, ex vivo and in vitro studies demonstrated preservation of muscle insulin responsiveness with no effect on adipose tissue or liver. Studies using neutralizing antibodies demonstrated a role for TNFα as mediator between myeloid Fas and skeletal muscle insulin resistance, supported by significant correlations between monocyte Fas expression and circulating TNFα in humans. In conclusion, our results demonstrate an unanticipated crosstalk between myeloid cells and skeletal muscle in the development of obesity-associated insulin resistance.
Figures





References
-
- Araujo EP, Souza De CT, Ueno M, Cintra DE, Bertolo MB, Carvalheira JB, Saad MJ, Velloso LA. Infliximab restores glucose homeostasis in an animal model of diet-induced obesity and diabetes. Endocrinology. 2007;148:5991–5997. - PubMed
-
- Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11:191–198. - PubMed
-
- Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. - PubMed
-
- Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8:265–277. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

