Recent advances in experimental techniques to probe fast excited-state dynamics in biological molecules in the gas phase: dynamics in nucleotides, amino acids and beyond
- PMID: 24204191
- PMCID: PMC3780818
- DOI: 10.1098/rspa.2013.0458
Recent advances in experimental techniques to probe fast excited-state dynamics in biological molecules in the gas phase: dynamics in nucleotides, amino acids and beyond
Abstract
In many chemical reactions, an activation barrier must be overcome before a chemical transformation can occur. As such, understanding the behaviour of molecules in energetically excited states is critical to understanding the chemical changes that these molecules undergo. Among the most prominent reactions for mankind to understand are chemical changes that occur in our own biological molecules. A notable example is the focus towards understanding the interaction of DNA with ultraviolet radiation and the subsequent chemical changes. However, the interaction of radiation with large biological structures is highly complex, and thus the photochemistry of these systems as a whole is poorly understood. Studying the gas-phase spectroscopy and ultrafast dynamics of the building blocks of these more complex biomolecules offers the tantalizing prospect of providing a scientifically intuitive bottom-up approach, beginning with the study of the subunits of large polymeric biomolecules and monitoring the evolution in photochemistry as the complexity of the molecules is increased. While highly attractive, one of the main challenges of this approach is in transferring large, and in many cases, thermally labile molecules into vacuum. This review discusses the recent advances in cutting-edge experimental methodologies, emerging as excellent candidates for progressing this bottom-up approach.
Keywords: dynamics; spectroscopy; ultrafast.
Figures

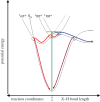
 (red) along ring-distortion coordinates. Evidence also exists for a
(red) along ring-distortion coordinates. Evidence also exists for a  pathway leading either to H-atom elimination (blue) or IC down to S0 (purple) along X−H bonds where X=O or N. CIs mediating non-radiative pathways are circled where appropriate (adapted from [–20]).
pathway leading either to H-atom elimination (blue) or IC down to S0 (purple) along X−H bonds where X=O or N. CIs mediating non-radiative pathways are circled where appropriate (adapted from [–20]).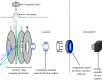

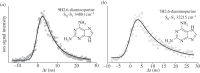


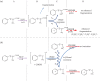


References
-
- Eberhard S, Finazzi G, Wollman FA. 2008. The dynamics of photosynthesis. Annu. Rev. Genet. 42, 463–515 (doi:10.1146/annurev.genet.42.110807.091452) - DOI - PubMed
-
- Collini E, Wong CY, Wilk KE, Curmi PM, Brumer P, Scholes GD. 2010. Coherently wired light-harvesting in photosynthetic marine algae at ambient temperature. Nature 463, 644–677 (doi:10.1038/nature08811) - DOI - PubMed
-
- Lee H, Cheng YC, Fleming GR. 2007. Coherence dynamics in photosynthesis: protein protection of excitonic coherence. Science 316, 1462–1465 (doi:10.1126/science.1142188) - DOI - PubMed
-
- Cheng YC, Fleming GR. 2009. Dynamics of light harvesting in photosynthesis. Annu. Rev. Phys. Chem. 60, 241–262 (doi:10.1146/annurev.physchem.040808.090259) - DOI - PubMed
-
- Arnett DC, Moser CC, Dutton PL, Scherer NF. 1999. The first events in photosynthesis: electronic coupling and energy transfer dynamics in the photosynthetic reaction center from Rhodobacter sphaeroides. J. Phys. Chem. B 103, 2014–2032 (doi:10.1021/jp984464j) - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

