Allosteric MEK1/2 inhibitor refametinib (BAY 86-9766) in combination with sorafenib exhibits antitumor activity in preclinical murine and rat models of hepatocellular carcinoma
- PMID: 24204195
- PMCID: PMC3819632
- DOI: 10.1593/neo.13812
Allosteric MEK1/2 inhibitor refametinib (BAY 86-9766) in combination with sorafenib exhibits antitumor activity in preclinical murine and rat models of hepatocellular carcinoma
Abstract
Objective: The objectives of the study were to evaluate the allosteric mitogen-activated protein kinase kinase (MEK) inhibitor BAY 86-9766 in monotherapy and in combination with sorafenib in orthotopic and subcutaneous hepatocellular carcinoma (HCC) models with different underlying etiologies in two species.
Design: Antiproliferative potential of BAY 86-9766 and synergistic effects with sorafenib were studied in several HCC cell lines. Relevant pathway signaling was studied in MH3924a cells. For in vivo testing, the HCC cells were implanted subcutaneously or orthotopically. Survival and mode of action (MoA) were analyzed.
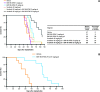
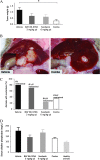
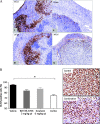
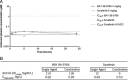
Results: BAY 86-9766 exhibited potent antiproliferative activity in HCC cell lines with half-maximal inhibitory concentration values ranging from 33 to 762 nM. BAY 86-9766 was strongly synergistic with sorafenib in suppressing tumor cell proliferation and inhibiting phosphorylation of the extracellular signal-regulated kinase (ERK). BAY 86-9766 prolonged survival in Hep3B xenografts, murine Hepa129 allografts, and MH3924A rat allografts. Additionally, tumor growth, ascites formation, and serum alpha-fetoprotein levels were reduced. Synergistic effects in combination with sorafenib were shown in Huh-7, Hep3B xenografts, and MH3924A allografts. On the signaling pathway level, the combination of BAY 86-9766 and sorafenib led to inhibition of the upregulatory feedback loop toward MEK phosphorylation observed after BAY 86-9766 monotreatment. With regard to the underlying MoA, inhibition of ERK phosphorylation, tumor cell proliferation, and microvessel density was observed in vivo.
Conclusion: BAY 86-9766 shows potent single-agent antitumor activity and acts synergistically in combination with sorafenib in preclinical HCC models. These results support the ongoing clinical development of BAY 86-9766 and sorafenib in advanced HCC.
Figures


References
-
- Venook AP, Papandreou C, Furuse J, de Guevara LL. The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. Oncologist. 2010;15(suppl 4):5–13. - PubMed
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. - PubMed
-
- Ma YT, Palmer DH. Impact of restricting access to high-cost medications for hepatocellular carcinoma. Expert Rev Pharmacoecon Outcomes Res. 2012;12:465–473. - PubMed
-
- Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–3310. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
