Plasmacytoid dendritic cells contribute to systemic but not local antiviral responses to HSV infections
- PMID: 24204273
- PMCID: PMC3812046
- DOI: 10.1371/journal.ppat.1003728
Plasmacytoid dendritic cells contribute to systemic but not local antiviral responses to HSV infections
Abstract
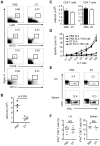
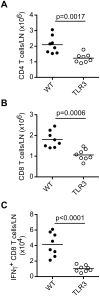
Plasmacytoid dendritic cells (pDC) produce type I interferons (IFN-I) and proinflammatory cytokines in response to viruses; however, their contribution to antiviral immunity in vivo is unclear. In this study, we investigated the impact of pDC depletion on local and systemic antiviral responses to herpes simplex virus (HSV) infections using CLEC4C-DTR transgenic mice. We found that pDC do not appear to influence viral burden or survival after vaginal HSV-2 infection, nor do they seem to contribute to virus-specific CD8 T cell responses following subcutaneous HSV-1 infection. In contrast, pDC were important for early IFN-I production, proinflammatory cytokine production, NK cell activation and CD8 T cell responses during systemic HSV-2 and HSV-1 infections. Our data also indicate that unlike pDC, TLR3-expressing cells are important for promoting antiviral responses to HSV-1 regardless of the route of virus administration.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

