The transcription factors Sox10 and Myrf define an essential regulatory network module in differentiating oligodendrocytes
- PMID: 24204311
- PMCID: PMC3814293
- DOI: 10.1371/journal.pgen.1003907
The transcription factors Sox10 and Myrf define an essential regulatory network module in differentiating oligodendrocytes
Abstract
Myelin is essential for rapid saltatory conduction and is produced by Schwann cells in the peripheral nervous system and oligodendrocytes in the central nervous system. In both cell types the transcription factor Sox10 is an essential component of the myelin-specific regulatory network. Here we identify Myrf as an oligodendrocyte-specific target of Sox10 and map a Sox10 responsive enhancer to an evolutionarily conserved element in intron 1 of the Myrf gene. Once induced, Myrf cooperates with Sox10 to implement the myelination program as evident from the physical interaction between both proteins and the synergistic activation of several myelin-specific genes. This is strongly reminiscent of the situation in Schwann cells where Sox10 first induces and then cooperates with Krox20 during myelination. Our analyses indicate that the regulatory network for myelination in oligodendrocytes is organized along similar general principles as the one in Schwann cells, but is differentially implemented.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


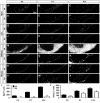

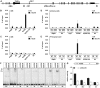


Comment in
-
Playing the field: Sox10 recruits different partners to drive central and peripheral myelination.PLoS Genet. 2013 Oct;9(10):e1003918. doi: 10.1371/journal.pgen.1003918. Epub 2013 Oct 31. PLoS Genet. 2013. PMID: 24204318 Free PMC article. No abstract available.
References
-
- Fröb F, Bremer M, Finzsch M, Kichko T, Reeh P, et al. (2012) Establishment of myelinating Schwann cells and barrier integrity between central and peripheral nervous systems depend on Sox10. Glia 60: 809–816. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

