The chemistry of isoindole natural products
- PMID: 24204418
- PMCID: PMC3817534
- DOI: 10.3762/bjoc.9.243
The chemistry of isoindole natural products
Abstract
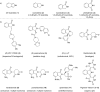
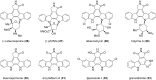
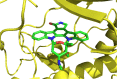
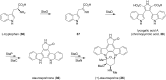
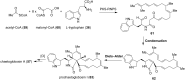
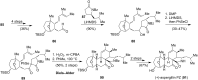
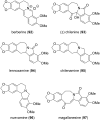
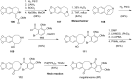
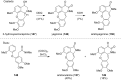
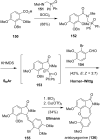
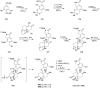
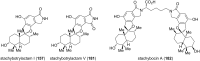
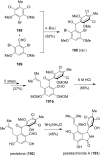
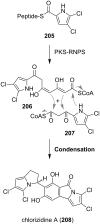
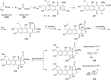
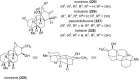
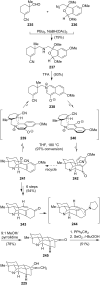
This review highlights the chemical and biological aspects of natural products containing an oxidized or reduced isoindole skeleton. This motif is found in its intact or modified form in indolocarbazoles, macrocyclic polyketides (cytochalasan alkaloids), the aporhoeadane alkaloids, meroterpenoids from Stachybotrys species and anthraquinone-type alkaloids. Concerning their biological activity, molecular structure and synthesis, we have limited this review to the most inspiring examples. Within different congeners, we have selected a few members and discussed the synthetic routes in more detail. The putative biosynthetic pathways of the presented isoindole alkaloids are described as well.
Keywords: isoindole; isoindoline; isoindolinone; isoindolone; natural products.
Figures













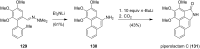
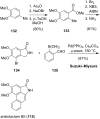
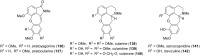
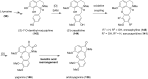







References
-
- Subbarayappa A, Patoliya P U. Indian J Chem, Sect B: Org Chem Incl Med Chem. 2009;48:545–552.
-
- Ball M, Boyd A, Churchill G, Cuthbert M, Drew M, Fielding M, Ford G, Frodsham L, Golden M, Leslie K, et al. Org Process Res Dev. 2012;16:741–747. doi: 10.1021/op300002f. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
