Enterocyte proliferation and signaling are constitutively altered in celiac disease
- PMID: 24204586
- PMCID: PMC3799793
- DOI: 10.1371/journal.pone.0076006
Enterocyte proliferation and signaling are constitutively altered in celiac disease
Abstract
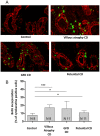
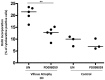
Celiac disease (CD) occurs frequently, and is caused by ingestion of prolamins from cereals in subjects with a genetic predisposition. The small intestinal damage depends on an intestinal stress/innate immune response to certain gliadin peptides (e.g., A-gliadin P31-43) in association with an adaptive immune response to other gliadin peptides (e.g., A-gliadin P57-68). Gliadin and peptide P31-43 affect epithelial growth factor receptor (EGFR) signaling and CD enterocyte proliferation. The reason why the stress/innate immune and proliferative responses to certain gliadin peptides are present in CD and not in control intestine is so far unknown. The aim of this work is to investigate if, in CD, a constitutive alteration of enterocyte proliferation and signaling exists that may represent a predisposing condition to the damaging effects of gliadin. Immunofluorescence and immunohistochemistry were used to study signaling in CD fibroblasts and intestinal biopsies. Western blot (WB) analysis, immunoprecipitation, and quantitative PCR were also used. We found in CD enterocytes enhancement of both proliferation and Epidermal Growth Factor Receptor (EGFR)/ligand system. In CD enterocytes and fibroblasts we found increase of the phosphorylated downstream signaling molecule Extracellular Signal Regulated Kinase (ERK); block of the ERK activation normalizes enterocytes proliferation in CD mucosa. In conclusion the same pathway, which gliadin and gliadin peptide P31-43 can interfere with, is constitutively altered in CD cells. This observation potentially explains the specificity of the damaging effects of certain gliadin peptides on CD intestine.
Conflict of interest statement
Figures



References
-
- Sollid LM (2000) Molecular basis of celiac disease. Annu Rev Immunol 18: 53–81. - PubMed
-
- Jabri B, Sollid LM (2006) Mechanisms of disease. Immuno-pathogenesis of Celiac disease. Nat Clin Pract Gastoenterol Hepathol 3: 516–525. - PubMed
-
- Abadie V, Sollid L, Barreiro LB, Jabri B (2010) Integration of genetic and immunological insights into a model of Celiac Disease pathogenesis. Annual Rev Immunol 29: 493–526. - PubMed
-
- Maiuri L, Ciacci C, Ricciardelli I, Vacca L, Raia V, et al. (2003) Association between innate response to gliadin and activation of pathogenic T cells in coeliac disease. Lancet 362: 30–37. - PubMed
-
- Hue S, Mention JJ, Monteiro RC, Zhang S, Cellier C, et al. (2004) A direct role for NKG2D/MICA interaction in villous atrophy during celiac disease. Immunity 21: 367–77. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

