Neural mechanisms influencing interlimb coordination during locomotion in humans: presynaptic modulation of forearm H-reflexes during leg cycling
- PMID: 24204611
- PMCID: PMC3799938
- DOI: 10.1371/journal.pone.0076313
Neural mechanisms influencing interlimb coordination during locomotion in humans: presynaptic modulation of forearm H-reflexes during leg cycling
Abstract
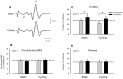
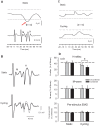
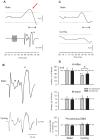
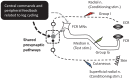
Presynaptic inhibition of transmission between Ia afferent terminals and alpha motoneurons (Ia PSI) is a major control mechanism associated with soleus H-reflex modulation during human locomotion. Rhythmic arm cycling suppresses soleus H-reflex amplitude by increasing segmental Ia PSI. There is a reciprocal organization in the human nervous system such that arm cycling modulates H-reflexes in leg muscles and leg cycling modulates H-reflexes in forearm muscles. However, comparatively little is known about mechanisms subserving the effects from leg to arm. Using a conditioning-test (C-T) stimulation paradigm, the purpose of this study was to test the hypothesis that changes in Ia PSI underlie the modulation of H-reflexes in forearm flexor muscles during leg cycling. Subjects performed leg cycling and static activation while H-reflexes were evoked in forearm flexor muscles. H-reflexes were conditioned with either electrical stimuli to the radial nerve (to increase Ia PSI; C-T interval = 20 ms) or to the superficial radial (SR) nerve (to reduce Ia PSI; C-T interval = 37-47 ms). While stationary, H-reflex amplitudes were significantly suppressed by radial nerve conditioning and facilitated by SR nerve conditioning. Leg cycling suppressed H-reflex amplitudes and the amount of this suppression was increased with radial nerve conditioning. SR conditioning stimulation removed the suppression of H-reflex amplitude resulting from leg cycling. Interestingly, these effects and interactions on H-reflex amplitudes were observed with subthreshold conditioning stimulus intensities (radial n., ∼0.6×MT; SR n., ∼ perceptual threshold) that did not have clear post synaptic effects. That is, did not evoke reflexes in the surface EMG of forearm flexor muscles. We conclude that the interaction between leg cycling and somatosensory conditioning of forearm H-reflex amplitudes is mediated by modulation of Ia PSI pathways. Overall our results support a conservation of neural control mechanisms between the arms and legs during locomotor behaviors in humans.
Conflict of interest statement
Figures


Similar articles
-
Effect of rhythmic arm movement on reflexes in the legs: modulation of soleus H-reflexes and somatosensory conditioning.J Neurophysiol. 2004 Apr;91(4):1516-23. doi: 10.1152/jn.00695.2003. Epub 2003 Dec 3. J Neurophysiol. 2004. PMID: 14657191
-
Facilitation of soleus H-reflex amplitude evoked by cutaneous nerve stimulation at the wrist is not suppressed by rhythmic arm movement.Exp Brain Res. 2004 Dec;159(3):382-8. doi: 10.1007/s00221-004-2092-x. Epub 2004 Oct 8. Exp Brain Res. 2004. PMID: 15480593
-
Rhythmic arm cycling differentially modulates stretch and H-reflex amplitudes in soleus muscle.Exp Brain Res. 2011 Oct;214(4):529-37. doi: 10.1007/s00221-011-2851-4. Epub 2011 Sep 8. Exp Brain Res. 2011. PMID: 21901451
-
Contributions to the understanding of gait control.Dan Med J. 2014 Apr;61(4):B4823. Dan Med J. 2014. PMID: 24814597 Review.
-
Differential effects of low-frequency depression, vibration-induced inhibition, and posttetanic potentiation on H-reflexes and tendon jerks in the human soleus muscle.J Neurophysiol. 1986 Mar;55(3):551-68. doi: 10.1152/jn.1986.55.3.551. J Neurophysiol. 1986. PMID: 3514814 Review.
Cited by
-
Sherlock Holmes and the curious case of the human locomotor central pattern generator.J Neurophysiol. 2018 Jul 1;120(1):53-77. doi: 10.1152/jn.00554.2017. Epub 2018 Mar 14. J Neurophysiol. 2018. PMID: 29537920 Free PMC article. Review.
-
D1 and D2 Inhibitions of the Soleus H-Reflex Are Differentially Modulated during Plantarflexion Force and Position Tasks.PLoS One. 2015 Nov 24;10(11):e0143862. doi: 10.1371/journal.pone.0143862. eCollection 2015. PLoS One. 2015. PMID: 26599909 Free PMC article. Clinical Trial.
-
Soleus Hoffmann reflex amplitudes are specifically modulated by cutaneous inputs from the arms and opposite leg during walking but not standing.Exp Brain Res. 2016 Aug;234(8):2293-304. doi: 10.1007/s00221-016-4635-3. Epub 2016 Mar 31. Exp Brain Res. 2016. PMID: 27030502
-
Phase-dependent reversal of the crossed conditioning effect on the soleus Hoffmann reflex from cutaneous afferents during walking in humans.Exp Brain Res. 2016 Feb;234(2):617-26. doi: 10.1007/s00221-015-4463-x. Epub 2015 Nov 16. Exp Brain Res. 2016. PMID: 26573576
-
Effects of a compression garment on sensory feedback transmission in the human upper limb.J Neurophysiol. 2018 Jul 1;120(1):186-195. doi: 10.1152/jn.00581.2017. Epub 2018 Apr 11. J Neurophysiol. 2018. PMID: 29641310 Free PMC article.
References
-
- Calancie B, Needham-Shropshire B, Jacobs P, Willer K, Zych G, et al. (1994) Involuntary stepping after chronic spinal cord injury. Evidence for a central rhythm generator for locomotion in man. Brain 117: 1143–1159. - PubMed
-
- Dietz V (2002) Do human bipeds use quadrupedal coordination? Trends Neurosci 25: 462–467. - PubMed
-
- Zehr EP, Duysens J (2004) Regulation of arm and leg movement during human locomotion. Neuroscientist 10: 347–361. - PubMed
-
- Sakamoto M, Tazoe T, Nakajima T, Endoh T, Shiozawa S, et al. (2007) Voluntary changes in leg cadence modulate arm cadence during simultaneous arm and leg cycling. Exp Brain Res 176: 188–192. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

