Extracellular vesicles derived from gut microbiota, especially Akkermansia muciniphila, protect the progression of dextran sulfate sodium-induced colitis
- PMID: 24204633
- PMCID: PMC3811976
- DOI: 10.1371/journal.pone.0076520
Extracellular vesicles derived from gut microbiota, especially Akkermansia muciniphila, protect the progression of dextran sulfate sodium-induced colitis
Abstract
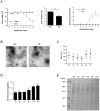
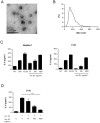
Gut microbiota play an important part in the pathogenesis of mucosal inflammation, such as inflammatory bowel disease (IBD). However, owing to the complexity of the gut microbiota, our understanding of the roles of commensal and pathogenic bacteria in the maintenance of immune homeostasis in the gut is evolving only slowly. Here, we evaluated the role of gut microbiota and their secreting extracellular vesicles (EV) in the development of mucosal inflammation in the gut. Experimental IBD model was established by oral application of dextran sulfate sodium (DSS) to C57BL/6 mice. The composition of gut microbiota and bacteria-derived EV in stools was evaluated by metagenome sequencing using bacterial common primer of 16S rDNA. Metagenomics in the IBD mouse model showed that the change in stool EV composition was more drastic, compared to the change of bacterial composition. Oral DSS application decreased the composition of EV from Akkermansia muciniphila and Bacteroides acidifaciens in stools, whereas increased EV from TM7 phylum, especially from species DQ777900_s and AJ400239_s. In vitro pretreatment of A. muciniphila-derived EV ameliorated the production of a pro-inflammatory cytokine IL-6 from colon epithelial cells induced by Escherichia coli EV. Additionally, oral application of A. muciniphila EV also protected DSS-induced IBD phenotypes, such as body weight loss, colon length, and inflammatory cell infiltration of colon wall. Our data provides insight into the role of gut microbiota-derived EV in regulation of intestinal immunity and homeostasis, and A. muciniphila-derived EV have protective effects in the development of DSS-induced colitis.
Conflict of interest statement
Figures




References
-
- Bouma G, Strober W (2003) The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol 3: 521–533. - PubMed
-
- Xavier RJ, Podolsky DK (2007) Unravelling the pathogenesis of inflammatory bowel disease. Nature 448: 427–434. - PubMed
-
- Hooper LV, Gordon JI (2001) Commensal host-bacterial relationships in the gut. Science 292: 1115–1118. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

