Essential role of the m2R-RGS6-IKACh pathway in controlling intrinsic heart rate variability
- PMID: 24204714
- PMCID: PMC3812209
- DOI: 10.1371/journal.pone.0076973
Essential role of the m2R-RGS6-IKACh pathway in controlling intrinsic heart rate variability
Abstract
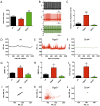
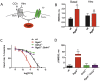
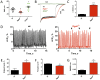
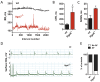
Normal heart function requires generation of a regular rhythm by sinoatrial pacemaker cells and the alteration of this spontaneous heart rate by the autonomic input to match physiological demand. However, the molecular mechanisms that ensure consistent periodicity of cardiac contractions and fine tuning of this process by autonomic system are not completely understood. Here we examined the contribution of the m2R-I(KACh) intracellular signaling pathway, which mediates the negative chronotropic effect of parasympathetic stimulation, to the regulation of the cardiac pacemaking rhythm. Using isolated heart preparations and single-cell recordings we show that the m2R-I(KACh) signaling pathway controls the excitability and firing pattern of the sinoatrial cardiomyocytes and determines variability of cardiac rhythm in a manner independent from the autonomic input. Ablation of the major regulator of this pathway, Rgs6, in mice results in irregular cardiac rhythmicity and increases susceptibility to atrial fibrillation. We further identify several human subjects with variants in the RGS6 gene and show that the loss of function in RGS6 correlates with increased heart rate variability. These findings identify the essential role of the m2R-I(KACh) signaling pathway in the regulation of cardiac sinus rhythm and implicate RGS6 in arrhythmia pathogenesis.
Conflict of interest statement
Figures
References
-
- Podrid PJ, Kowey PR (2001) Cardiac Arrhythmia: Mechanisms, Diagnosis, and Management: Lippincott Williams & Wilkins. 973 p.
-
- Mangoni ME, Nargeot J (2008) Genesis and regulation of the heart automaticity. Physiol Rev 88: 919–982. - PubMed
-
- Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger AC, et al. (1981) Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science 213: 220–222. - PubMed
-
- Gehrmann J, Hammer PE, Maguire CT, Wakimoto H, Triedman JK, et al. (2000) Phenotypic screening for heart rate variability in the mouse. Am J Physiol Heart Circ Physiol 279: H733–740. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

