Safety and immunogenicity of a live attenuated RSV vaccine in healthy RSV-seronegative children 5 to 24 months of age
- PMID: 24204744
- PMCID: PMC3812203
- DOI: 10.1371/journal.pone.0077104
Safety and immunogenicity of a live attenuated RSV vaccine in healthy RSV-seronegative children 5 to 24 months of age
Abstract
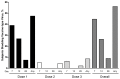
Despite substantial morbidity associated with respiratory syncytial virus (RSV) infection, there is no licensed vaccine. MEDI-559 is a live attenuated intranasal vaccine candidate being developed for prevention of lower respiratory illness due to RSV in young children. This randomized, placebo-controlled study evaluated safety of MEDI-559 in healthy, RSV-seronegative children. MEDI-559 or placebo was administered on 3 occasions, 2 months apart. Primary safety was based on solicited symptoms (SSs) and adverse events (AEs) collected for 28 days after each dose. Nasal wash samples were collected 3 times after each dose (days 7-10, 12-18, 28-34) and at sick visits. Serum was collected for measuring antibody immune responses to RSV prior to first vaccination and 28 days post final dose. Long-term safety was monitored for 365 days from first dose. SSs were mild and frequent (MEDI-559 84%; placebo 91%); most common SSs were runny/stuffy nose, cough, and irritability/fussiness. AEs occurred in 67% MEDI-559 and 57% placebo recipients: most common AE was upper respiratory tract infection (MEDI-559 35%; placebo 23%). Higher incidence of medically attended lower respiratory illness within 28 days after dosing occurred in the MEDI-559 arm compared to placebo (none associated with vaccine virus shedding). There was no evidence of enhanced RSV disease. Vaccine virus was detected only in MEDI-559 recipients; shedding occurred in 56%subjects, primarily post dose 1. A functional immune response was observed in 59% and 9% MEDI-559 and placebo recipients, respectively, by an RSV microneutralization assay. Vaccine take, assessed by proportion that shed vaccine-type virus or had a seroresponse against RSV, was seen in 95% MEDI-559 subjects. MEDI-559 is therefore biologically active and immunogenic in this seronegative pediatric population. Although the frequency of SSs and AEs was not considered clinically significant, the increase in medically attended lower respiratory illnesses in the vaccine group warrants expanded safety studies.
Trial registration: ClinicalTrials.gov NCT00767416.
Conflict of interest statement
Figures
References
-
- Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA et al. (2010) Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: A systematic review and meta-analysis. Lancet 375: 1545-1555. doi:10.1016/S0140-6736(10)60206-1. PubMed: 20399493. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical