Benefits of therapeutic drug monitoring of vancomycin: a systematic review and meta-analysis
- PMID: 24204764
- PMCID: PMC3799644
- DOI: 10.1371/journal.pone.0077169
Benefits of therapeutic drug monitoring of vancomycin: a systematic review and meta-analysis
Abstract
Background and objective: The necessity of therapeutic drug monitoring (TDM) for vancomycin is controversial. The objective of the current review was to evaluate the available evidence for the necessity of TDM in patients given vancomycin to treat Gram-positive infections.
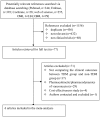
Methods: Medline, Embase, Web of Sciences, the Cochrane Library and two Chinese literature databases (CNKI, CBM) were searched. Randomized controlled studies and observational studies that compared the clinical outcomes of TDM groups vs. non-TDM groups were included. Two reviewers independently extracted the data. The primary outcome was clinical efficacy of therapy. Secondary outcomes included vancomycin associated nephrotoxicity, duration of vancomycin therapy, length of hospital stay, and mortality. Meta-analysis was performed using the Mantel-Haenszel fixed effect method (FEM). Odds ratios (ORs) or weighted mean differences (WMD) with 95% confidence intervals (95%CIs) were calculated for categorical and continuous outcomes, respectively.
Results: One randomized controlled trial (RCT) and five cohort studies were included in the meta-analysis. Compared with non-TDM groups, TDM groups had significantly higher rates of clinical efficacy (OR = 2.62, 95%CI 1.34-5.11 P = 0.005) and decreased rates of nephrotoxicity (OR = 0.25, 95%CI 0.13-0.48 P<0.0001). Subgroup analyses showed that TDM group had significantly higher rates of clinical efficacy in both cohort studies subgroup (OR = 3.04, 95%CI 1.34-6.90) and in Asian population subgroup (OR = 3.04, 95%CI 1.34-6.90). TDM group had significantly decreased rates of nephrotoxicity in all subgroup. There was no significant difference in duration of vancomycin therapy (WMD = -0.40, 95%CI -2.83-2.02 P = 0.74) or length of stay (WMD = -1.01, 95%CI -7.51-5.49 P = 0.76) between TDM and non-TDM groups. Subgroup analyses showed there were no differences in duration of vancomycin therapy. Only one study reported mortality rates.
Conclusions: Studies to date show that TDM significantly increases the rate of clinical efficacy and decreases the rate of nephrotoxicity in patients treated with vancomycin.
Conflict of interest statement
Figures






References
-
- Cataldo MA, Tacconelli E, Grilli E, Pea F, Petrosillo N (2012) Continuous versus intermittent infusion of vancomycin for the treatment of Gram-positive infections: systematic review and meta-analysis. J Antimicrob Chemother 67: 17–24. - PubMed
-
- Moellering RC Jr (2006) Vancomycin: a 50-year reassessment. Clin Infect Dis 42 Suppl 1: S3–4. - PubMed
-
- Levine DP (2006) Vancomycin: a history. Clin Infect Dis 42 Suppl 1: S5–12. - PubMed
-
- Rybak MJ (2006) The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin Infect Dis 42 Suppl 1: S35–39. - PubMed
-
- Darko W, Medicis JJ, Smith A, Guharoy R, Lehmann DE (2003) Mississippi mud no more: cost-effectiveness of pharmacokinetic dosage adjustment of vancomycin to prevent nephrotoxicity. Pharmacotherapy 23: 643–650. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

