Effect of Iboga alkaloids on µ-opioid receptor-coupled G protein activation
- PMID: 24204784
- PMCID: PMC3818563
- DOI: 10.1371/journal.pone.0077262
Effect of Iboga alkaloids on µ-opioid receptor-coupled G protein activation
Abstract
Objective: The iboga alkaloids are a class of small molecules defined structurally on the basis of a common ibogamine skeleton, some of which modify opioid withdrawal and drug self-administration in humans and preclinical models. These compounds may represent an innovative approach to neurobiological investigation and development of addiction pharmacotherapy. In particular, the use of the prototypic iboga alkaloid ibogaine for opioid detoxification in humans raises the question of whether its effect is mediated by an opioid agonist action, or if it represents alternative and possibly novel mechanism of action. The aim of this study was to independently replicate and extend evidence regarding the activation of μ-opioid receptor (MOR)-related G proteins by iboga alkaloids.
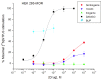
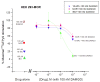
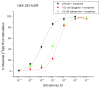
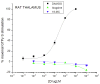
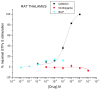
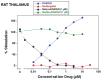
Methods: Ibogaine, its major metabolite noribogaine, and 18-methoxycoronaridine (18-MC), a synthetic congener, were evaluated by agonist-stimulated guanosine-5´-O-(γ-thio)-triphosphate ([(35)S]GTPγS) binding in cells overexpressing the recombinant MOR, in rat thalamic membranes, and autoradiography in rat brain slices.
Results and significance: In rat thalamic membranes ibogaine, noribogaine and 18-MC were MOR antagonists with functional Ke values ranging from 3 uM (ibogaine) to 13 uM (noribogaine and 18MC). Noribogaine and 18-MC did not stimulate [(35)S]GTPγS binding in Chinese hamster ovary cells expressing human or rat MORs, and had only limited partial agonist effects in human embryonic kidney cells expressing mouse MORs. Ibogaine did not did not stimulate [(35)S]GTPγS binding in any MOR expressing cells. Noribogaine did not stimulate [(35)S]GTPγS binding in brain slices using autoradiography. An MOR agonist action does not appear to account for the effect of these iboga alkaloids on opioid withdrawal. Taken together with existing evidence that their mechanism of action also differs from that of other non-opioids with clinical effects on opioid tolerance and withdrawal, these findings suggest a novel mechanism of action, and further justify the search for alternative targets of iboga alkaloids.
Conflict of interest statement
Figures


References
-
- Bartlett MF, Dickel DF, Taylor WI (1958) The alkaloids of Tabernanthe-Iboga. Part VI. The Structures of ibogamine, ibogaine, tabernanthine and voacangine. J Am Chem Soc 80: 126-136. doi:10.1021/ja01534a036. - DOI
-
- Jana GK, Paul S, Sinha S (2011) Progress in the synthesis of iboga-alkaloids and their congeners. Org Prep Proced Int 43: 541-573. doi:10.1080/00304948.2011.629563. - DOI
-
- Fernandez JW (1982) Bwiti: An Ethnography of Religious Imagination in Africa. Princeton, New Jersey: Princeton University Press. 731 pp.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

