Nitric oxide synthase-3 promotes embryonic development of atrioventricular valves
- PMID: 24204893
- PMCID: PMC3812218
- DOI: 10.1371/journal.pone.0077611
Nitric oxide synthase-3 promotes embryonic development of atrioventricular valves
Abstract
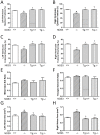
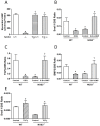
Nitric oxide synthase-3 (NOS3) has recently been shown to promote endothelial-to-mesenchymal transition (EndMT) in the developing atrioventricular (AV) canal. The present study was aimed to investigate the role of NOS3 in embryonic development of AV valves. We hypothesized that NOS3 promotes embryonic development of AV valves via EndMT. To test this hypothesis, morphological and functional analysis of AV valves were performed in wild-type (WT) and NOS3(-/-) mice at postnatal day 0. Our data show that the overall size and length of mitral and tricuspid valves were decreased in NOS3(-/-) compared with WT mice. Echocardiographic assessment showed significant regurgitation of mitral and tricuspid valves during systole in NOS3(-/-) mice. These phenotypes were all rescued by cardiac specific NOS3 overexpression. To assess EndMT, immunostaining of Snail1 was performed in the embryonic heart. Both total mesenchymal and Snail1(+) cells in the AV cushion were decreased in NOS3(-/-) compared with WT mice at E10.5 and E12.5, which was completely restored by cardiac specific NOS3 overexpression. In cultured embryonic hearts, NOS3 promoted transforming growth factor (TGFβ), bone morphogenetic protein (BMP2) and Snail1expression through cGMP. Furthermore, mesenchymal cell formation and migration from cultured AV cushion explants were decreased in the NOS3(-/-) compared with WT mice. We conclude that NOS3 promotes AV valve formation during embryonic heart development and deficiency in NOS3 results in AV valve insufficiency.
Conflict of interest statement
Figures





Similar articles
-
Bone morphogenetic protein-2 can mediate myocardial regulation of atrioventricular cushion mesenchymal cell formation in mice.Dev Biol. 2004 May 15;269(2):505-18. doi: 10.1016/j.ydbio.2004.01.045. Dev Biol. 2004. PMID: 15110716
-
BMP2 expression in the endocardial lineage is required for AV endocardial cushion maturation and remodeling.Dev Biol. 2017 Oct 1;430(1):113-128. doi: 10.1016/j.ydbio.2017.08.008. Epub 2017 Aug 6. Dev Biol. 2017. PMID: 28790014 Free PMC article.
-
Transforming growth factor β, bone morphogenetic protein, and vascular endothelial growth factor mediate phenotype maturation and tissue remodeling by embryonic valve progenitor cells: relevance for heart valve tissue engineering.Tissue Eng Part A. 2010 Nov;16(11):3375-83. doi: 10.1089/ten.tea.2010.0027. Epub 2010 Jul 14. Tissue Eng Part A. 2010. PMID: 20629541 Free PMC article.
-
NOing the heart: role of nitric oxide synthase-3 in heart development.Differentiation. 2012 Jul;84(1):54-61. doi: 10.1016/j.diff.2012.04.004. Epub 2012 May 11. Differentiation. 2012. PMID: 22579300 Review.
-
Mechanisms involved in valvuloseptal endocardial cushion formation in early cardiogenesis: roles of transforming growth factor (TGF)-beta and bone morphogenetic protein (BMP).Anat Rec. 2000 Feb 1;258(2):119-27. doi: 10.1002/(SICI)1097-0185(20000201)258:2<119::AID-AR1>3.0.CO;2-U. Anat Rec. 2000. PMID: 10645959 Review.
Cited by
-
Is NO the Answer? The Nitric Oxide Pathway Can Support Bone Morphogenetic Protein 2 Mediated Signaling.Cells. 2019 Oct 18;8(10):1273. doi: 10.3390/cells8101273. Cells. 2019. PMID: 31635347 Free PMC article.
-
Bicuspid aortic valve formation: Nos3 mutation leads to abnormal lineage patterning of neural crest cells and the second heart field.Dis Model Mech. 2018 Oct 19;11(10):dmm034637. doi: 10.1242/dmm.034637. Dis Model Mech. 2018. PMID: 30242109 Free PMC article.
-
Sapropterin Treatment Prevents Congenital Heart Defects Induced by Pregestational Diabetes Mellitus in Mice.J Am Heart Assoc. 2018 Nov 6;7(21):e009624. doi: 10.1161/JAHA.118.009624. J Am Heart Assoc. 2018. PMID: 30608180 Free PMC article.
-
Krox20 Regulates Endothelial Nitric Oxide Signaling in Aortic Valve Development and Disease.J Cardiovasc Dev Dis. 2019 Nov 2;6(4):39. doi: 10.3390/jcdd6040039. J Cardiovasc Dev Dis. 2019. PMID: 31684048 Free PMC article.
-
Rac1 signaling is critical to cardiomyocyte polarity and embryonic heart development.J Am Heart Assoc. 2014 Oct 14;3(5):e001271. doi: 10.1161/JAHA.114.001271. J Am Heart Assoc. 2014. PMID: 25315346 Free PMC article.
References
-
- Siu SC, Silversides CK (2010) Bicuspid aortic valve disease. J Am Coll Cardiol 55: 2789–2800. - PubMed
-
- Horne BD, Camp NJ, Muhlestein JB, Cannon-Albright LA (2004) Evidence for a heritable component in death resulting from aortic and mitral valve diseases. Circulation 110: 3143–3148. - PubMed
-
- Glick BN, Roberts WC (1994) Congenitally bicuspid aortic valve in multiple family members. Am J Cardiol 73: 400–404. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

