Interobserver agreement of Thyroid Imaging Reporting and Data System (TIRADS) and strain elastography for the assessment of thyroid nodules
- PMID: 24205031
- PMCID: PMC3812025
- DOI: 10.1371/journal.pone.0077927
Interobserver agreement of Thyroid Imaging Reporting and Data System (TIRADS) and strain elastography for the assessment of thyroid nodules
Abstract
Background: Thyroid Imaging Reporting and Data System (TIRADS) was developed to improve patient management and cost-effectiveness by avoiding unnecessary fine needle aspiration biopsy (FNAB) in patients with thyroid nodules. However, its clinical use is still very limited. Strain elastography (SE) enables the determination of tissue elasticity and has shown promising results for the differentiation of thyroid nodules.
Methods: The aim of the present study was to evaluate the interobserver agreement (IA) of TIRADS developed by Horvath et al. and SE. Three blinded observers independently scored stored images of TIRADS and SE in 114 thyroid nodules (114 patients). Cytology and/or histology was available for all benign (n = 99) and histology for all malignant nodules (n = 15).
Results: The IA between the 3 observers was only fair for TIRADS categories 2-5 (Coheńs kappa = 0.27,p = 0.000001) and TIRADS categories 2/3 versus 4/5 (ck = 0.25,p = 0.0020). The IA was substantial for SE scores 1-4 (ck = 0.66,p<0.000001) and very good for SE scores 1/2 versus 3/4 (ck = 0.81,p<0.000001). 92-100% of patients with TIRADS-2 had benign lesions, while 28-42% with TIRADS-5 had malignant cytology/histology. The negative-predictive-value (NPV) was 92-100% for TIRADS using TIRADS-categories 4&5 and 96-98% for SE using score ES-3&4 for the diagnosis of malignancy, respectively. However, only 11-42% of nodules were in TIRADS-categories 2&3, as compared to 58-60% with ES-1&2.
Conclusions: IA of TIRADS developed by Horvath et al. is only fair. TIRADS and SE have high NPV for excluding malignancy in the diagnostic work-up of thyroid nodules.
Conflict of interest statement
Figures
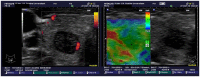
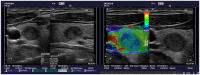
Comment on
-
An ultrasonogram reporting system for thyroid nodules stratifying cancer risk for clinical management.J Clin Endocrinol Metab. 2009 May;94(5):1748-51. doi: 10.1210/jc.2008-1724. Epub 2009 Mar 10. J Clin Endocrinol Metab. 2009. PMID: 19276237
References
-
- Reiners C, Wegscheider K, Schicha H, Theissen P, Vaupel R, et al. (2004) Prevalence of thyroid disorders in the working population of Germany: ultrasonography screening in 96,278 unselected employees. Thyroid 14: 926–932. - PubMed
-
- Iannuccilli JD, Cronan JJ, Monchik JM (2004) Risk for malignancy of thyroid nodules as assessed by sonographic criteria: the need for biopsy. J Ultrasound Med 23: 1455–1464. - PubMed
-
- Dietlein M, Schicha H (2003) Lifetime follow-up care is necessary for all patients with treated thyroid nodules. Eur J Endocrinol 148: 377–379. - PubMed
-
- Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, et al. (2006) Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 16: 109–142. - PubMed
-
- Gharib H, Papini E, Paschke R, Duick DS, Valcavi R, et al. (2010) American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and EuropeanThyroid Association Medical Guidelines for Clinical Practice for the Diagnosis and Management of Thyroid Nodules. Endocr Pract 16 Suppl 11–43. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

