Aqueous extract of Bambusae Caulis in Taeniam inhibits PMA-induced tumor cell invasion and pulmonary metastasis: suppression of NF-κB activation through ROS signaling
- PMID: 24205091
- PMCID: PMC3810142
- DOI: 10.1371/journal.pone.0078061
Aqueous extract of Bambusae Caulis in Taeniam inhibits PMA-induced tumor cell invasion and pulmonary metastasis: suppression of NF-κB activation through ROS signaling
Abstract
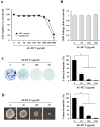
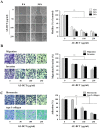
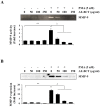
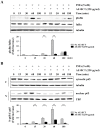
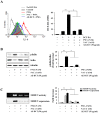
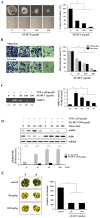
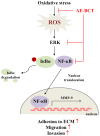
Bamboo shavings (Bambusae Caulis in Taeniam, BCT) are widely used as a traditional Chinese medicine to control hypertension and cardiovascular disease, and to alleviate fever, vomiting, and diarrhea. It has been demonstrated that BCT reduces ovalbumin-induced airway inflammation by regulating pro-inflammatory cytokines, and decreases tumor growth in tumor-bearing mice. However, the effects of BCT on the metastatic potential of malignant cancer cells and the detailed mechanism of its anti-metastatic activity have not been examined previously. In this study, we investigated whether an aqueous extract of BCT (AE-BCT) reduces the metastatic potential of HT1080 cells, and elucidated the underlying anti-metastatic mechanism. In addition, we examined whether AE-BCT administration inhibits pulmonary metastasis of intravenously injected B16F10 cells in C57BL/6J mice. AE-BCT (50-250 µg/ml) dose-dependently suppressed colony-forming activity under anchorage-dependent and -independent growth conditions. Pretreatment with AE-BCT efficiently inhibited cell migration, invasion, and adhesion. AE-BCT also dramatically suppressed PMA-induced MMP-9 activity and expression by blocking NF-κB activation and ERK phosphorylation. Production of intracellular ROS, a key regulator of NF-κB-induced MMP-9 activity, was almost completely blocked by pretreatment with AE-BCT. Furthermore, daily oral administration of AE-BCT at doses of 50 and 100 mg/kg efficiently inhibited lung metastasis of B16F10 cells injected into the tail veins of C57BL/6J mice with no systemic toxicity. These results demonstrate that AE-BCT significantly reduced the metastatic activity of highly malignant cancer cells by suppressing MMP-9 activity via inhibition of ROS-mediated NF-κB activation. These results indicate that AE-BCT may be a safe natural product for treatment of metastatic cancer.
Conflict of interest statement
Figures

References
-
- Liotta LA, Steeg PS, Stetler-Stevenson WG (1991) Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell 64: 327–336. - PubMed
-
- Chambers AF, Groom AC, MacDonald IC (2002) Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer 2: 563–572. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

