Adult-specific systemic over-expression reveals novel in vivo effects of the soluble forms of ActRIIA, ActRIIB and BMPRII
- PMID: 24205096
- PMCID: PMC3804470
- DOI: 10.1371/journal.pone.0078076
Adult-specific systemic over-expression reveals novel in vivo effects of the soluble forms of ActRIIA, ActRIIB and BMPRII
Abstract
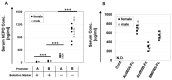
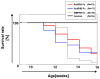
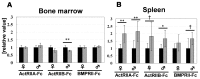
Bone morphogenetic proteins (BMPs)/growth differentiation factors (GDFs), which belong to the TGF-beta superfamily, are pleiotropic factors that play a role in regulating the embryonic development and postnatal homeostasis of various organs and tissues by controlling cellular differentiation, proliferation and apoptosis. Conventional transgenic and knockout (KO) mouse approaches have provided only limited information regarding the in vivo functions of BMP signaling in adult animals due to the effects on prenatal development and the difficulty in manipulating multiligand signals simultaneously. We recently produced transgenic chimeric mice(Tg chimeras) in which the soluble IgG1-Fc fusion protein of three BMP type II receptors (ActRIIA, ActRIIB, BMPRII) was highly circulated (281-709 μg/ml), specifically in adult mouse blood. Since each BMP receptor can bind to multiple BMP ligands, these Tg chimeras should be useful to investigate the effects of trapping multiple BMP ligands. Remarkably, some phenotypes were unexpected based on previous studies, such as KO mouse analyses, presumably representing the effects of the multiple ligand trapping. These phenotypes included increased red blood cells (RBCs) and decreased viability in adults. In a further study, we focused on the phenotype of increased RBCs and found that extramedullary hematopoiesis in the spleen, not in the bone marrow, was increased using histological and flow cytometric analyses. Although it remains to be elucidated whether the transgene products affect the tissues directly or indirectly, our data provide novel and important insight into the biological functions of the soluble IgG1-Fc fusion protein of three BMP type II receptors in adults, and our approach should have broad applications to research on other ligand receptor families and studies involving mouse models.
Conflict of interest statement
Figures


References
-
- Kitisin K, Saha T, Blake T, Golestaneh N, Deng M et al. (2007) Tgf-beta signaling in development. Sci STKE 2007: cm1 PubMed: 17699101 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

