Duration of HIV-1 viral suppression on cessation of antiretroviral therapy in primary infection correlates with time on therapy
- PMID: 24205183
- PMCID: PMC3808338
- DOI: 10.1371/journal.pone.0078287
Duration of HIV-1 viral suppression on cessation of antiretroviral therapy in primary infection correlates with time on therapy
Abstract
Objective: A minority of HIV-1 positive individuals treated with antiretroviral therapy (ART) in primary HIV-1 infection (PHI) maintain viral suppression on stopping. Whether this is related to ART duration has not been explored.
Design: And Methods: Using SPARTAC trial data from individuals recruited within 6 months of seroconversion, we present an observational analysis investigating whether duration of ART was associated with post-treatment viraemic control. Kaplan-Meier estimates, logistic regression and Cox models were used.
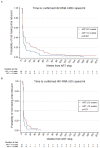
Results: 165 participants reached plasma viral loads (VL) <400 copies/ml at the time of stopping therapy (ART stop). After ART stop, 159 experienced confirmed VL ≥400 copies/ml during median (IQR) follow-up of 167 (108,199) weeks. Most participants experienced VL rebound within 12 weeks from ART stop, however, there was a suggestion of a higher probability of remaining <400 copies/ml for those on ART >12 weeks compared to ≤12 weeks (p=0.061). Cumulative probabilities of remaining <400 copies/ml at 12, 52 and 104 weeks after ART stop were 21% (95%CI=13,30), 4% (1,9), and 4% (1,9) for ≤12 weeks ART, and 32% (22,42), 14% (7,22), and 5% (2,11) for >12 weeks. In multivariable regression, ART for >12 weeks was independently associated with a lower probability of being ≥400 copies/ml within 12 weeks of ART stop (OR=0.11 (95%CI=0.03,0.34), p<0.001)). In Cox models of time to VL ≥400 after 12 weeks, we only found an association with female sex (OR=0.2, p=0.001).
Conclusion: Longer ART duration in PHI was associated with a higher probability of viral control after ART stop.
Trial registration: Controlled-Trials.com 76742797 http://www.controlled-trials.com/ISRCTN76742797.
Conflict of interest statement
Figures
References
-
- Hocqueloux L, Prazuck T, Avettand-Fenoel V, Lafeuillade A, Cardon B et al. (2010) Long-term immunovirologic control following antiretroviral therapy interruption in patients treated at the time of primary HIV-1 infection. AIDS 24: 1598–1601. doi: 10.1097/QAD.0b013e32833b61ba. PubMed: 20549847. - DOI - PubMed
-
- Goujard C, Girault I, Rouzioux C, Lécuroux C, Deveau C et al. (2012) HIV-1 control after transient antiretroviral treatment initiated in primary infection: role of patient characteristics and effect of therapy. Antivir Ther (Lond) 17: 1001–1009. doi: 10.3851/IMP2273. PubMed: 22865544. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

