Circadian clock control of Nox4 and reactive oxygen species in the vasculature
- PMID: 24205282
- PMCID: PMC3808297
- DOI: 10.1371/journal.pone.0078626
Circadian clock control of Nox4 and reactive oxygen species in the vasculature
Abstract
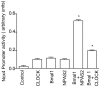
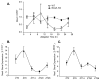
Recent studies have shown that circadian clock disruption is associated with pathological remodeling in the arterial structure and vascular stiffness. Moreover, chronic circadian disruption is associated with dysfunction in endothelial responses and signaling. Reactive oxygen species have emerged as key regulators in vascular pathology. Previously, we have demonstrated that circadian clock dysfunction exacerbates superoxide production through eNOS uncoupling. To date, the impact of circadian clock mutation on vascular NADPH oxidase expression and function is not known. The goal in the current study was to determine if the circadian clock controls vascular Nox4 expression and hydrogen peroxide formation in arteries, particularly in endothelial and vascular smooth muscle cells. In aorta, there was an increase in hydrogen peroxide and Nox4 expression in mice with a dysfunctional circadian rhythm (Bmal1-KO mice). In addition, the Nox4 gene promoter is activated by the core circadian transcription factors. Lastly, in synchronized cultured human endothelial cells, Nox4 gene expression exhibited rhythmic oscillations. These data reveal that the circadian clock plays an important role in the control of Nox4 and disruption of the clock leads to subsequent production of reaction oxygen species.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

