Aqueous ethanolic extract of Tinospora cordifolia as a potential candidate for differentiation based therapy of glioblastomas
- PMID: 24205314
- PMCID: PMC3811968
- DOI: 10.1371/journal.pone.0078764
Aqueous ethanolic extract of Tinospora cordifolia as a potential candidate for differentiation based therapy of glioblastomas
Abstract
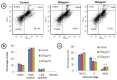
Glioblastomas are the most aggressive primary brain tumors and their heterogeneity and complexity often renders them non responsive to various conventional treatments. Search for herbal products having potential anti-cancer activity is an active area of research in the Indian traditional system of medicine i.e., Ayurveda. Tinospora cordifolia, also named as 'heavenly elixir' is used in various ayurvedic decoctions as panacea to treat several body ailments. The current study investigated the anti-brain cancer potential of 50% ethanolic extract of Tinospora cordifolia (TCE) using C6 glioma cells. TCE significantly reduced cell proliferation in dose-dependent manner and induced differentiation in C6 glioma cells, resulting in astrocyte-like morphology as indicated by phase contrast images, GFAP expression and process outgrowth data of TCE treated cells which exhibited higher number and longer processes than untreated cells. Reduced proliferation of cells was accompanied by enhanced expression of senescence marker, mortalin and its translocation from perinuclear to pancytoplasmic spaces. Further, TCE showed anti-migratory and anti-invasive potential as depicted by wound scratch assay and reduced expression of plasticity markers NCAM and PSA-NCAM along with MMP-2 and 9. On analysis of the cell cycle and apoptotic markers, TCE treatment was seen to arrest the C6 cells in G0/G1 and G2/M phase, suppressing expression of G1/S phase specific protein cyclin D1 and anti-apoptotic protein Bcl-xL, thus supporting its anti-proliferative and apoptosis inducing potential. Present study provides the first evidence for the presence of anti-proliferative, differentiation-inducing and anti-migratory/anti-metastatic potential of TCE in glioma cells and possible signaling pathways involved in its mode of action. Our primary data suggests that TCE and its active components may prove to be promising phytotherapeutic interventions in gliobalstoma multiformae.
Conflict of interest statement
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

