Acute physiological stress promotes clustering of synaptic markers and alters spine morphology in the hippocampus
- PMID: 24205365
- PMCID: PMC3812005
- DOI: 10.1371/journal.pone.0079077
Acute physiological stress promotes clustering of synaptic markers and alters spine morphology in the hippocampus
Abstract
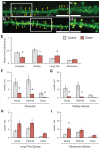
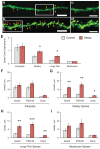
GluA2-containing AMPA receptors and their association with protein kinase M zeta (PKMζ) and post-synaptic density-95 (PSD-95) are important for learning, memory and synaptic plasticity processes. Here we investigated these synaptic markers in the context of an acute 1h platform stress, which can disrupt spatial memory retrieval for a short-term memory on the object placement task and long-term memory retrieval on a well-learned radial arm maze task. Acute stress increased serum corticosterone and elevated the expression of synaptic PKMζ while decreasing synaptic GluA2. Using co-immunoprecipitation, we found that this stressor promotes the clustering of GluA2, PKMζ and PSD-95, which is consistent with effects reported from overexpression of PKMζ in cell culture. Because PKMζ overexpression has also been shown to induce spine maturation in culture, we examined how stress impacts synaptic markers within changing spines across various hippocampal subfields. To achieve this, we employed a new technique combining Golgi staining and immmunohistochemistry to perform 3D reconstruction of tertiary dendrites, which can be analyzed for differences in spine types and the colocalization of synaptic markers within these spines. In CA1, stress increased the densities of long-thin and mushroom spines and the colocalization of GluA2/PSD-95 within these spines. Conversely, in CA3, stress decreased the densities of filopodia and stubby spines, with a concomitant reduction in the colocalization of GluA2/PSD-95 within these spines. In the outer molecular layer (OML) of the dentate gyrus (DG), stress increased both stubby and long-thin spines, together with greater GluA2/PSD-95 colocalization. These data reflect the rapid effects of stress on inducing morphological changes within specific hippocampal subfields, highlighting a potential mechanism by which stress can modulate memory consolidation and retrieval.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

