A Biohybrid Device for the Systemic Control of Acute Inflammation
- PMID: 24205448
- PMCID: PMC3817839
- DOI: 10.1089/dst.2012.0001
A Biohybrid Device for the Systemic Control of Acute Inflammation
Abstract
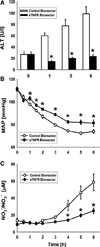
Properly regulated inflammation facilitates recognition and reaction to injury or infection, but inadequate or overly robust inflammation can lead to disease. Sepsis is an inflammatory disease that accounts for nearly 10% of total U.S. deaths, costing more than $17 billion. Acute inflammation in sepsis may evolve too rapidly to be modulated appropriately, and we suggest that therapies should focus not on abolishing inflammation, but rather on attenuating the positive feedback cycle of inflammation/damage/inflammation. In Gram-negative sepsis, bacterial endotoxin causes inflammation and is driven and regulated by the cytokine tumor necrosis factor-α (TNF-α), which is, in turn, negatively regulated via its endogenous inhibitor, soluble TNF-α receptor (sTNFR). We generated stably gene-modified variants of human HepG2 hepatocytes, using lentiviral constructs coding for mouse sTNFR driven by the constitutive cytomegalovirus promoter, and seeded them in a scaled-down, experimental liver bioreactor. When connected to anesthetized, cannulated rats subjected to endotoxin infusion and maintained solely by the animals' circulation, this biohybrid device elevated circulating sTNFR, reduced the levels of TNF-α and other key inflammatory mediators, alleviated hypotension, and reduced circulating markers of organ damage. This novel class of biohybrid devices may bemodified for patient- and disease-specific application, and, thus, may represent a disruptive strategy that offers the potential for rational inflammation reprogramming.
Keywords: biohybrid device; endotoxin; multiple organ dysfunction syndrome; sepsis; soluble tumor necrosis factor-alpha receptor 1; tumor necrosis factor alpha.
Figures



References
-
- Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. - PubMed
-
- Martin GS, Mannino DM, Eaton S, et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. - PubMed
-
- Vincent JL, Sakr Y, Sprung CL, et al. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34:344–353. - PubMed
-
- Moore FA, Moore EE, Sauaia A. In: Postinjury multiple-organ failure in Trauma. Mattox KL, Feliciano DV, Moore EE, editors. New York, NY: McGraw-Hill; 1999. pp. 1427–1459.
-
- Angus DC, Wax RS. The epidemiology of sepsis: An update. Crit Care Med. 2001;29(Suppl 7):S109–S116. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
