Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease
- PMID: 24206459
- PMCID: PMC4496027
- DOI: 10.1056/NEJMoa1306033
Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease
Abstract
Background: Although inhibitors of the renin-angiotensin-aldosterone system can slow the progression of diabetic kidney disease, the residual risk is high. Whether nuclear 1 factor (erythroid-derived 2)-related factor 2 activators further reduce this risk is unknown.
Methods: We randomly assigned 2185 patients with type 2 diabetes mellitus and stage 4 chronic kidney disease (estimated glomerular filtration rate [GFR], 15 to <30 ml per minute per 1.73 m(2) of body-surface area) to bardoxolone methyl, at a daily dose of 20 mg, or placebo. The primary composite outcome was end-stage renal disease (ESRD) or death from cardiovascular causes.
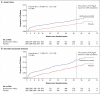
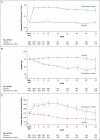
Results: The sponsor and the steering committee terminated the trial on the recommendation of the independent data and safety monitoring committee; the median follow-up was 9 months. A total of 69 of 1088 patients (6%) randomly assigned to bardoxolone methyl and 69 of 1097 (6%) randomly assigned to placebo had a primary composite outcome (hazard ratio in the bardoxolone methyl group vs. the placebo group, 0.98; 95% confidence interval [CI], 0.70 to 1.37; P=0.92). In the bardoxolone methyl group, ESRD developed in 43 patients, and 27 patients died from cardiovascular causes; in the placebo group, ESRD developed in 51 patients, and 19 patients died from cardiovascular causes. A total of 96 patients in the bardoxolone methyl group were hospitalized for heart failure or died from heart failure, as compared with 55 in the placebo group (hazard ratio, 1.83; 95% CI, 1.32 to 2.55; P<0.001). Estimated GFR, blood pressure, and the urinary albumin-to-creatinine ratio increased significantly and body weight decreased significantly in the bardoxolone methyl group, as compared with the placebo group.
Conclusions: Among patients with type 2 diabetes mellitus and stage 4 chronic kidney disease, bardoxolone methyl did not reduce the risk of ESRD or death from cardiovascular causes. A higher rate of cardiovascular events with bardoxolone methyl than with placebo prompted termination of the trial. (Funded by Reata Pharmaceuticals; BEACON ClinicalTrials.gov number, NCT01351675.).
Figures

Comment in
-
New therapies for diabetic kidney disease.N Engl J Med. 2013 Dec 26;369(26):2549-50. doi: 10.1056/NEJMe1313104. Epub 2013 Nov 9. N Engl J Med. 2013. PMID: 24206460 No abstract available.
-
Bardoxolone methyl in type 2 diabetes and advanced chronic kidney disease.N Engl J Med. 2014 May 1;370(18):1768. doi: 10.1056/NEJMc1400872. N Engl J Med. 2014. PMID: 24785220 No abstract available.
-
Bardoxolone methyl in type 2 diabetes and advanced chronic kidney disease.N Engl J Med. 2014 May 1;370(18):1767. doi: 10.1056/NEJMc1400872. N Engl J Med. 2014. PMID: 24785221 No abstract available.
-
Bardoxolone methyl in type 2 diabetes and advanced chronic kidney disease.N Engl J Med. 2014 May 1;370(18):1767-8. doi: 10.1056/NEJMc1400872. N Engl J Med. 2014. PMID: 24785222 No abstract available.
-
The paradox of bardoxolone methyl: a call for every witness on the stand?Diabetes Obes Metab. 2015 Jan;17(1):9-14. doi: 10.1111/dom.12356. Epub 2014 Aug 4. Diabetes Obes Metab. 2015. PMID: 25041694
-
The paradox created by commenting on large clinical trial results.Diabetes Obes Metab. 2015 Jan;17(1):1-2. doi: 10.1111/dom.12392. Diabetes Obes Metab. 2015. PMID: 25243381 No abstract available.
References
-
- Klahr S, Levey AS, Beck GJ, et al. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. N Engl J Med. 1994;330:877–84. - PubMed
-
- The ADVANCE Collaborative Group Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–72. - PubMed
-
- Parving HH, Andersen AR, Smidt UM, Svendsen PA. Early aggressive antihypertensive treatment reduces rate of decline in kidney function in diabetic nephropathy. Lancet. 1983;1:1175–9. - PubMed
-
- Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–9. - PubMed
-
- Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–60. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
