Evaluation of parasite subpopulations and genetic diversity of the msp1, msp2 and glurp genes during and following artesunate monotherapy treatment of Plasmodium falciparum malaria in Western Cambodia
- PMID: 24206588
- PMCID: PMC3830508
- DOI: 10.1186/1475-2875-12-403
Evaluation of parasite subpopulations and genetic diversity of the msp1, msp2 and glurp genes during and following artesunate monotherapy treatment of Plasmodium falciparum malaria in Western Cambodia
Abstract
Background: Despite widespread coverage of the emergence of artemisinin resistance, relatively little is known about the parasite populations responsible. The use of PCR genotyping around the highly polymorphic Plasmodium falciparum msp1, msp2 and glurp genes has become well established both to describe variability in alleles within a population of parasites, as well as classify treatment outcome in cases of recurrent disease. The primary objective was to assess the emergence of minority parasite clones during seven days of artesunate (AS) treatment in a location with established artemisinin resistance. An additional objective was to investigate whether the classification of clinical outcomes remained valid when additional genotyping was performed.
Methods: Blood for parasite genotyping was collected from 143 adult patients presenting with uncomplicated falciparum malaria during a clinical trial of AS monotherapy in Western Cambodia. Nested allelic type-specific amplification of the genes encoding the merozoite surface proteins 1 and 2 (msp1 and msp2) and the glutamate-rich protein (glurp) was performed at baseline, daily during seven days of treatment, and again at failure. Allelic variants were analysed with respect to the size of polymorphisms using Quantity One software to enable identification of polyclonal infections.
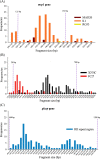
Results: Considerable variation of msp2 alleles but well-conserved msp1 and glurp were identified. At baseline, 31% of infections were polyclonal for one or more genes. Patients with recurrent malaria were significantly more likely to have polyclonal infections than patients without recurrence (seven of nine versus 36 of 127, p = 0.004). Emergence of minority alleles during treatment was detected in only one of twenty-three cases defined as being artemisinin resistant. Moreover, daily genotyping did not alter the final outcome classification in any recurrent cases.
Conclusions: The parasites responsible for artemisinin-resistant malaria in a clinical trial in Western Cambodia comprise the dominant clones of acute malaria infections rather than minority clones emerging during treatment. Additional genotyping during therapy was not beneficial. Disproportionately high rates of polyclonal infections in cases of recurrence suggest complex infections lead to poor treatment outcomes. Current research objectives should be broadened to include identification and follow-up of recurrent polyclonal infections so as to define their role as potential agents of emerging resistance.
Figures


References
-
- Phyo AP, Nkhoma S, Stepniewska K, Ashley EA, Nair S, McGready R, ler Moo C, Al-Saai S, Dondorp AM, Lwin KM, Singhasivanon P, Day NP, White NJ, Anderson TJ, Nosten F. Emergence of artemisinin-resistant malaria on the western border of Thailand: a longitudinal study. Lancet. 2012;379:1960–1966. doi: 10.1016/S0140-6736(12)60484-X. - DOI - PMC - PubMed
-
- WHO. Global Report on Antimalarial Drug Efficacy and Drug Resistance: 2000-2010. Geneva: World Health Organization; 2010.
-
- Miotto O, Almagro-Garcia J, Manske M, Macinnis B, Campino S, Rockett KA, Amaratunga C, Lim P, Suon S, Sreng S, Anderson JM, Duong S, Nguon C, Chuor CM, Saunders D, Se Y, Lon C, Fukuda MM, Amenga-Etego L, Hodgson AV, Asoala V, Imwong M, Takala-Harrison S, Nosten F, Su XZ, Ringwald P, Ariey F, Dolecek C, Hien TT, Boni MF. et al.Multiple populations of artemisinin-resistant Plasmodium falciparum in Cambodia. Nat Genet. 2013;45:648–655. doi: 10.1038/ng.2624. - DOI - PMC - PubMed
-
- Takala-Harrison S, Clark TG, Jacob CG, Cummings MP, Miotto O, Dondorp AM, Fukuda MM, Nosten F, Noedl H, Imwong M, Bethell D, Se Y, Lon C, Tyner SD, Saunders DL, Socheat D, Ariey F, Phyo AP, Starzengruber P, Fuehrer HP, Swoboda P, Stepniewska K, Flegg J, Arze C, Cerqueira GC, Silva JC, Ricklefs SM, Porcella SF, Stephens RM, Adams M. et al.Genetic loci associated with delayed clearance of Plasmodium falciparum following artemisinin treatment in Southeast Asia. Proc Natl Acad Sci U S A. 2013;110:240–245. doi: 10.1073/pnas.1211205110. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

