Elimination of gaseous microemboli from cardiopulmonary bypass using hypobaric oxygenation
- PMID: 24206970
- PMCID: PMC4294693
- DOI: 10.1016/j.athoracsur.2013.08.074
Elimination of gaseous microemboli from cardiopulmonary bypass using hypobaric oxygenation
Abstract
Background: Numerous gaseous microemboli (GME) are delivered into the arterial circulation during cardiopulmonary bypass (CPB). These emboli damage end organs through multiple mechanisms that are thought to contribute to neurocognitive deficits after cardiac surgery. Here, we use hypobaric oxygenation to reduce dissolved gases in blood and greatly reduce GME delivery during CPB.
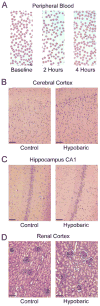
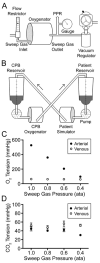
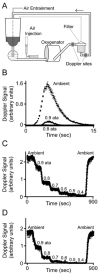
Methods: Variable subatmospheric pressures were applied to 100% oxygen sweep gas in standard hollow fiber microporous membrane oxygenators to oxygenate and denitrogenate blood. GME were quantified using ultrasound while air embolism from the surgical field was simulated experimentally. We assessed end-organ tissues in swine postoperatively using light microscopy.
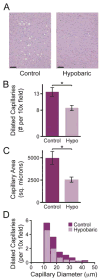
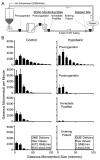
Results: Variable sweep gas pressures allowed reliable oxygenation independent of carbon dioxide removal while denitrogenating arterial blood. Hypobaric oxygenation produced dose-dependent reductions of Doppler signals produced by bolus and continuous GME loads in vitro. Swine were maintained using hypobaric oxygenation for 4 hours on CPB with no apparent adverse events. Compared with current practice standards of oxygen/air sweep gas, hypobaric oxygenation reduced GME volumes exiting the oxygenator (by 80%), exiting the arterial filter (95%), and arriving at the aortic cannula (∼100%), indicating progressive reabsorption of emboli throughout the CPB circuit in vivo. Analysis of brain tissue suggested decreased microvascular injury under hypobaric conditions.
Conclusions: Hypobaric oxygenation is an effective, low-cost, common sense approach that capitalizes on the simple physical makeup of GME to achieve their near-total elimination during CPB. This technique holds great potential for limiting end-organ damage and improving outcomes in a variety of patients undergoing extracorporeal circulation.
Copyright © 2014 The Society of Thoracic Surgeons. Published by Elsevier Inc. All rights reserved.
Figures
Comment in
-
Invited commentary.Ann Thorac Surg. 2014 Mar;97(3):887. doi: 10.1016/j.athoracsur.2013.09.035. Ann Thorac Surg. 2014. PMID: 24580906 No abstract available.
References
-
- Newman MF, Mathew JP, Grocott HP, et al. Central nervous system injury associated with cardiac surgery. Lancet. 2006;368:694–703. - PubMed
-
- Grocott HP, Homi HM, Puskas F. Cognitive dysfunction after cardiac surgery: revisiting etiology. Semin Cardiothorac Vasc Anesth. 2005;9:123–9. - PubMed
-
- Wang S, Woitas K, Clark JB, Myers JL, Undar A. Clinical real-time monitoring of gaseous microemboli in pediatric cardiopulmonary bypass. Artif Organs. 2009;33:1026–30. - PubMed
-
- Moody DM, Bell MA, Challa VR, Johnston WE, Prough DS. Brain microemboli during cardiac surgery or aortography. Ann Neurol. 1990;28:477–86. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

