The DNA translocase FANCM/MHF promotes replication traverse of DNA interstrand crosslinks
- PMID: 24207054
- PMCID: PMC3880019
- DOI: 10.1016/j.molcel.2013.09.021
The DNA translocase FANCM/MHF promotes replication traverse of DNA interstrand crosslinks
Abstract
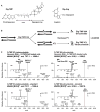
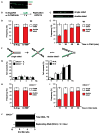
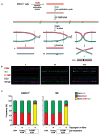
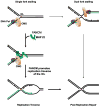
The replicative machinery encounters many impediments, some of which can be overcome by lesion bypass or replication restart pathways, leaving repair for a later time. However, interstrand crosslinks (ICLs), which preclude DNA unwinding, are considered absolute blocks to replication. Current models suggest that fork collisions, either from one or both sides of an ICL, initiate repair processes required for resumption of replication. To test these proposals, we developed a single-molecule technique for visualizing encounters of replication forks with ICLs as they occur in living cells. Surprisingly, the most frequent patterns were consistent with replication traverse of an ICL, without lesion repair. The traverse frequency was strongly reduced by inactivation of the translocase and DNA binding activities of the FANCM/MHF complex. The results indicate that translocase-based mechanisms enable DNA synthesis to continue past ICLs and that these lesions are not always absolute blocks to replication.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures



References
-
- Bagby GC, Alter BP. Fanconi anemia. Semin Hematol. 2006;43:147–156. - PubMed
-
- Bakker ST, van de Vrugt HJ, Rooimans MA, Oostra AB, Steltenpool J, Delzenne-Goette E, van der Wal A, van dV, Joenje H, te RH, de Winter JP. Fancm-deficient mice reveal unique features of Fanconi anemia complementation group M. Hum Mol Genet. 2009;18:3484–3495. - PubMed
-
- Blackford AN, Schwab RA, Nieminuszczy J, Deans AJ, West SC, Niedzwiedz W. The DNA translocase activity of FANCM protects stalled replication forks. Hum Mol Genet. 2012;21:2005–2016. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

