A zebrafish embryo culture system defines factors that promote vertebrate myogenesis across species
- PMID: 24209627
- PMCID: PMC3902670
- DOI: 10.1016/j.cell.2013.10.023
A zebrafish embryo culture system defines factors that promote vertebrate myogenesis across species
Abstract
Ex vivo expansion of satellite cells and directed differentiation of pluripotent cells to mature skeletal muscle have proved difficult challenges for regenerative biology. Using a zebrafish embryo culture system with reporters of early and late skeletal muscle differentiation, we examined the influence of 2,400 chemicals on myogenesis and identified six that expanded muscle progenitors, including three GSK3β inhibitors, two calpain inhibitors, and one adenylyl cyclase activator, forskolin. Forskolin also enhanced proliferation of mouse satellite cells in culture and maintained their ability to engraft muscle in vivo. A combination of bFGF, forskolin, and the GSK3β inhibitor BIO induced skeletal muscle differentiation in human induced pluripotent stem cells (iPSCs) and produced engraftable myogenic progenitors that contributed to muscle repair in vivo. In summary, these studies reveal functionally conserved pathways regulating myogenesis across species and identify chemical compounds that expand mouse satellite cells and differentiate human iPSCs into engraftable muscle.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
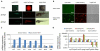
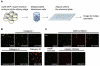



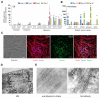
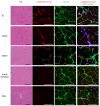
Comment in
-
Regenerative medicine: Of fish and men.Nat Chem Biol. 2014 Feb;10(2):91-2. doi: 10.1038/nchembio.1449. Nat Chem Biol. 2014. PMID: 24441638 No abstract available.
References
-
- Barberi T, Bradbury M, Dincer Z, Panagiotakos G, Socci ND, Studer L. Derivation of engraftable skeletal myoblasts from human embryonic stem cells. Nat Med. 2007a;13:642–648. - PubMed
-
- Barberi T, Bradbury M, Dincer Z, Panagiotakos G, Socci ND, Studer L. Derivation of engraftable skeletal myoblasts from human embryonic stem cells. Nature Medicine. 2007b;13:642–648. - PubMed
-
- Brack AS, Bildsoe H, Hughes SM. Evidence that satellite cell decrement contributes to preferential decline in nuclear number from large fibres during murine age-related muscle atrophy. J Cell Sci. 2005;118:4813–4821. - PubMed
-
- Brack AS, Conboy IM, Conboy MJ, Shen J, Rando TA. A temporal switch from notch to Wnt signaling in muscle stem cells is necessary for normal adult myogenesis. Cell Stem Cell. 2008;2:50–59. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 5R01CA103846-10/CA/NCI NIH HHS/United States
- P30 DK049216/DK/NIDDK NIH HHS/United States
- R01 CA103846/CA/NCI NIH HHS/United States
- DK31036/DK/NIDDK NIH HHS/United States
- R37 DK031036/DK/NIDDK NIH HHS/United States
- R01 DK031036/DK/NIDDK NIH HHS/United States
- R24DK092760-03/DK/NIDDK NIH HHS/United States
- R01 DK055545/DK/NIDDK NIH HHS/United States
- U01 HL100402/HL/NHLBI NIH HHS/United States
- 5U01HL10001-05/HL/NHLBI NIH HHS/United States
- P30 DK036836/DK/NIDDK NIH HHS/United States
- R01 HL048801/HL/NHLBI NIH HHS/United States
- DP2 OD004345/OD/NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R24 DK092760/DK/NIDDK NIH HHS/United States
- UO1HL100402/HL/NHLBI NIH HHS/United States
- 5P30 DK49216-19/DK/NIDDK NIH HHS/United States
- U54 AR052646/AR/NIAMS NIH HHS/United States
- DP2OD004345/OD/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

