KSR2 mutations are associated with obesity, insulin resistance, and impaired cellular fuel oxidation
- PMID: 24209692
- PMCID: PMC3898740
- DOI: 10.1016/j.cell.2013.09.058
KSR2 mutations are associated with obesity, insulin resistance, and impaired cellular fuel oxidation
Abstract
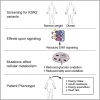
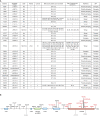
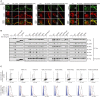
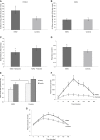
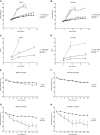
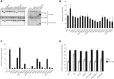
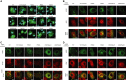
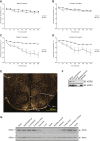
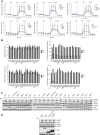
Kinase suppressor of Ras 2 (KSR2) is an intracellular scaffolding protein involved in multiple signaling pathways. Targeted deletion of Ksr2 leads to obesity in mice, suggesting a role in energy homeostasis. We explored the role of KSR2 in humans by sequencing 2,101 individuals with severe early-onset obesity and 1,536 controls. We identified multiple rare variants in KSR2 that disrupt signaling through the Raf-MEKERK pathway and impair cellular fatty acid oxidation and glucose oxidation in transfected cells; effects that can be ameliorated by the commonly prescribed antidiabetic drug, metformin. Mutation carriers exhibit hyperphagia in childhood, low heart rate, reduced basal metabolic rate and severe insulin resistance. These data establish KSR2 as an important regulator of energy intake, energy expenditure, and substrate utilization in humans. Modulation of KSR2-mediated effects may represent a novel therapeutic strategy for obesity and type 2 diabetes.
Figures



Comment in
-
Genetics: KSR2 mutations affect energy balance, insulin sensitivity and cellular fuel oxidation.Nat Rev Endocrinol. 2014 Jan;10(1):1. doi: 10.1038/nrendo.2013.228. Epub 2013 Nov 12. Nat Rev Endocrinol. 2014. PMID: 24217267 No abstract available.
-
Discovery of an obesity susceptibility gene, KSR2, provides new insight into energy homeostasis pathways.Circ Cardiovasc Genet. 2014 Apr;7(2):218-9. doi: 10.1161/CIRCGENETICS.114.000601. Circ Cardiovasc Genet. 2014. PMID: 24736852 No abstract available.
References
-
- Andersson U., Filipsson K., Abbott C.R., Woods A., Smith K., Bloom S.R., Carling D., Small C.J. AMP-activated protein kinase plays a role in the control of food intake. J. Biol. Chem. 2004;279:12005–12008. - PubMed
-
- Bost F., Aouadi M., Caron L., Even P., Belmonte N., Prot M., Dani C., Hofman P., Pagès G., Pouysségur J. The extracellular signal-regulated kinase isoform ERK1 is specifically required for in vitro and in vivo adipogenesis. Diabetes. 2005;54:402–411. - PubMed
-
- Brennan D.F., Dar A.C., Hertz N.T., Chao W.C., Burlingame A.L., Shokat K.M., Barford D. A Raf-induced allosteric transition of KSR stimulates phosphorylation of MEK. Nature. 2011;472:366–369. - PubMed
-
- Brommage R., Desai U., Revelli J.P., Donoviel D.B., Fontenot G.K., Dacosta C.M., Smith D.D., Kirkpatrick L.L., Coker K.J., Donoviel M.S. High-throughput screening of mouse knockout lines identifies true lean and obese phenotypes. Obesity (Silver Spring) 2008;16:2362–2367. - PubMed
-
- Canal F., Palygin O., Pankratov Y., Corrêa S.A., Müller J. Compartmentalization of the MAPK scaffold protein KSR1 modulates synaptic plasticity in hippocampal neurons. FASEB J. 2011;25:2362–2372. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MC_U106179471/MRC_/Medical Research Council/United Kingdom
- 096106/Z/11/Z/WT_/Wellcome Trust/United Kingdom
- MC_UU_12012/5/MRC_/Medical Research Council/United Kingdom
- MC_UP_1201/6/MRC_/Medical Research Council/United Kingdom
- 098497/Z/12/Z/WT_/Wellcome Trust/United Kingdom
- G0600717/MRC_/Medical Research Council/United Kingdom
- 14109/CRUK_/Cancer Research UK/United Kingdom
- MC_UU_12015/1/MRC_/Medical Research Council/United Kingdom
- WT091310/WT_/Wellcome Trust/United Kingdom
- G0900554/MRC_/Medical Research Council/United Kingdom
- 077016/Z/05/Z/WT_/Wellcome Trust/United Kingdom
- G0502115/MRC_/Medical Research Council/United Kingdom
- 098497/WT_/Wellcome Trust/United Kingdom
- 100574/WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

