Trends in hepatitis B virus screening at the onset of chemotherapy in a large US cancer center
- PMID: 24209764
- PMCID: PMC3827843
- DOI: 10.1186/1471-2407-13-534
Trends in hepatitis B virus screening at the onset of chemotherapy in a large US cancer center
Abstract
Background: National organizations recommend screening for hepatitis B virus (HBV) before chemotherapy but differ regarding which patients should be screened. We aimed to determine contemporary screening rates at a cancer center and the possible influence on these rates of publication of national recommendations.
Methods: We conducted a retrospective cohort study of HBV screening in cancer patients registered during the period from January 2004 through April 2011. Screening was defined as HBsAg and anti-HBc tests ordered around the time of initial chemotherapy. We compared screening rates for 3 periods: January 1, 2004, through December 18, 2008 (Food and Drug Administration and American Association for the Study of Liver Diseases 2007 recommendations); December 19, 2008, through September 30, 2010 (Centers for Disease Control and Prevention, National Comprehensive Cancer Network, American Association for the Study of Liver Diseases 2009, Institute of Medicine, and American Society of Clinical Oncology recommendations); and October 1, 2010, through April 30, 2011. Logistic regression models were used to identify predictors of screening.
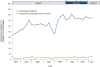
Results: Of 141,877 new patients, 18,688 received chemotherapy, and 3020 (16.2%) were screened. HBV screening rates increased over the 3 time periods (14.8%, 18.2%, 19.9%; P <0.0001), but <19% of patients with HBV risk factors were screened. Among patients with hematologic malignancies, over 66% were screened, and odds of screening nearly doubled after publication of the recommendations (P <0.0001). Less than 4% of patients with solid tumors were screened, although odds of screening increased 70% after publication of the recommendations (P =0.003). Other predictors of screening included younger age, planned rituximab therapy, and known risk factors for HBV infection.
Conclusions: Most patients with solid tumors or HBV risk factors remained unscreened, although screening rates increased after publication of national recommendations. Efforts are needed to increase awareness of the importance of HBV screening before chemotherapy to identify patients who should start antiviral prophylaxis.
Figures

References
-
- MedWatch safety information. Rituxan (rituximab) Oct 2004. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHuma...]. Access date: August 31, 2012.
-
- Weinbaum CM, Williams I, Mast EE, Wang SA, Finelli L, Wasley A, Neitzel SM, Ward JW. Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. MMWR Recomm Rep. 2008;57(RR-8):1–20. - PubMed
-
- Lok ASF, McMahon BJ. AASLD Practice Guideline Update: Chronic Hepatitis B: Update 2009. Available at: http://www.aasld.org/practiceguidelines/Documents/Bookmarked%20Practice%... Accessed November 7, 2013.?(2009)
-
- Practice guidelines in oncology. Prevention and treatment of cancer-related infections. 2009. http://www.nccn.org/professionals/physician_gls/pdf/infections.pdf]. Access date: August 31, 2012.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

