Development of a murine model of blunt hepatic trauma
- PMID: 24210016
- PMCID: PMC3796750
Development of a murine model of blunt hepatic trauma
Abstract
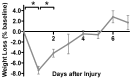
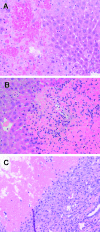
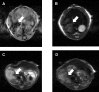
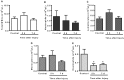
Despite the prevalence of blunt hepatic trauma in humans, there are few rodent models of blunt trauma that can be used to study the associated inflammatory responses. We present a mouse model of blunt hepatic trauma that was created by using a cortical contusion device. Male mice were anesthetized with ketamine-xylazine-buprenorphine and placed in left lateral recumbency. A position of 2 mm ventral to the posterior axillary line and 5 mm caudal to the costal margin on the right side was targeted for impact. An impact velocity of 6 m/s and a piston depth of 12 mm produced a consistent pattern of hepatic injury with low mortality. All mice that recovered from anesthesia survived without complication for the length of the study. Mice were euthanized at various time points (n = 5 per group) until 7 d after injury for gross examination and collection of blood and peritoneal lavage fluids. Some mice were reanesthetized for serial monitoring of hepatic lesions via MRI. At 2 h after trauma, mice consistently displayed laceration, hematoma, and discoloration of the right lateral and caudate liver lobes, with intraabdominal hemorrhage but no other gross injuries. Blood and peritoneal lavage fluid were collected from all mice for cytokine analysis. At 2 h after trauma, there were significant increases in plasma IL10 as well as peritoneal lavage fluid IL6 and CXCL1/KC; however, these levels decreased within 24 h. At 7 d after trauma, the mice had regained body weight, and the hepatic lesions, which initially had increased in size during the first 48 h, had returned to their original size. In summary, this technique produced a reliable, low mortality, murine model that recreates features of blunt abdominal liver injury in human subjects with similar acute inflammatory response.
Figures



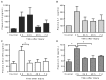


Similar articles
-
Cardiopulmonary, histological, and inflammatory alterations after lung contusion in a novel mouse model of blunt chest trauma.Shock. 2003 Jun;19(6):519-25. doi: 10.1097/01.shk.0000070739.34700.f6. Shock. 2003. PMID: 12785006
-
Hepatic venous injury after blunt abdominal trauma.Surgery. 1990 Feb;107(2):149-52. Surgery. 1990. PMID: 2300894
-
Hepatic injury.Acta Cir Bras. 2006;21 Suppl 1:85-8. doi: 10.1590/s0102-86502006000700019. Acta Cir Bras. 2006. PMID: 17013521 Review.
-
Concomitant hollow viscus injuries in patients with blunt hepatic and splenic injuries: an analysis of a National Trauma Registry database.Injury. 2014 Sep;45(9):1409-12. doi: 10.1016/j.injury.2014.02.027. Epub 2014 Feb 28. Injury. 2014. PMID: 24656303
-
Hepatic trauma: CT findings and considerations based on our experience in emergency diagnostic imaging.Eur J Radiol. 2004 Apr;50(1):59-66. doi: 10.1016/j.ejrad.2003.11.015. Eur J Radiol. 2004. PMID: 15093236 Review.
Cited by
-
Uncontrolled Hemorrhagic Shock Modeled via Liver Laceration in Mice with Real Time Hemodynamic Monitoring.J Vis Exp. 2017 May 21;(123):55554. doi: 10.3791/55554. J Vis Exp. 2017. PMID: 28570538 Free PMC article.
-
The impact of augmenter of liver regeneration in blunt liver trauma: An experimental model analysis.Ulus Travma Acil Cerrahi Derg. 2024 Jul;30(7):472-479. doi: 10.14744/tjtes.2024.92575. Ulus Travma Acil Cerrahi Derg. 2024. PMID: 38967532 Free PMC article.
-
The Role of Substance P in Pulmonary Clearance of Bacteria in Comparative Injury Models.Am J Pathol. 2016 Dec;186(12):3236-3245. doi: 10.1016/j.ajpath.2016.08.014. Am J Pathol. 2016. PMID: 27876152 Free PMC article.
-
Early Hyperbaric Oxygen Therapy Promotes Recovery of Blunt Liver Injury in Rats via Inhibiting Inflammatory Response and Oxidative Stress.Int J Med Sci. 2025 Apr 13;22(9):2174-2185. doi: 10.7150/ijms.109842. eCollection 2025. Int J Med Sci. 2025. PMID: 40303491 Free PMC article.
References
-
- Badger SA, Barclay R, Campbell P, Mole DJ, Diamond T. 2009. Management of liver trauma. World J Surg 33:2522–2537 - PubMed
-
- Camprodon R, Solsona J, Guerrero JA, Mendoza CG, Segura J, Fabregat JM. 1977. Intrahepatic vascular division in the pig: basis for partial hepatectomies. Arch Surg 112:38–40 - PubMed
-
- Gebhard F, Pfetsch H, Steinbach G, Strecker W, Kinzl L, Bruckner UB. 2000. Is interleukin 6 an early marker of injury severity following major trauma in humans? Arch Surg 135:291–295 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
