A randomised trial of electro-acupuncture for arthralgia related to aromatase inhibitor use
- PMID: 24210070
- PMCID: PMC3972040
- DOI: 10.1016/j.ejca.2013.09.022
A randomised trial of electro-acupuncture for arthralgia related to aromatase inhibitor use
Abstract
Background: Arthralgia is a common and debilitating side-effect experienced by breast cancer patients receiving aromatase inhibitors (AIs) and often results in premature drug discontinuation.
Methods: We conducted a randomised controlled trial of electro-acupuncture (EA) as compared to waitlist control (WLC) and sham acupuncture (SA) in postmenopausal women with breast cancer who self-reported arthralgia attributable to AIs. Acupuncturists performed 10 EA/SA treatments over 8 weeks using a manualised protocol with 2 Hz electro-stimulation delivered by a TENS unit. Acupuncturists administered SA using Streitberger (non-penetrating) needles at non-traditional acupuncture points without electro-stimulation. The primary end-point was pain severity by Brief Pain Inventory (BPI) between EA and WLC at Week 8; durability of response at Week 12 and comparison of EA to SA were secondary aims.
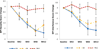
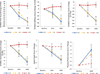
Findings: Of the 67 randomly assigned patients, mean reduction in pain severity was greater in the EA group than in the WLC group at Week 8 (-2.2 versus -0.2, p=0.0004) and at Week 12 (-2.4 versus -0.2, p<0.0001). Pain-related interference measured by BPI also improved in the EA group compared to the WLC group at both Week 8 (-2.0 versus 0.2, p=0.0006) and Week 12 (-2.1 versus -0.1, p=0.0034). SA produced a magnitude of change in pain severity and pain-related interference at Week 8 (-2.3, -1.5 respectively) and Week 12 (-1.7, -1.3 respectively) similar to that of EA. Participants in both EA and SA groups reported few minor adverse events.
Interpretations: Compared to usual care, EA produced clinically important and durable improvement in arthralgia related to AIs in breast cancer patients, and SA had a similar effect. Both EA and SA were safe.
Trial registration: ClinicalTrials.gov NCT01013337.
Keywords: Acupuncture; Adverse effects; Aromatase inhibitors; Breast neoplasm; Clinical trial; Joint pain; Musculoskeletal.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Dr. Mao has consulted for Pfizer on issues unrelated to aromatase inhibitors. Dr. Farrar has consulted for Pfizer and AstaZeneca on issues that are unrelated to aromatase inhibitors. The other co-authors had no conflict of interest to declare.
Figures
Similar articles
-
Effect of Acupuncture vs Sham Acupuncture or Waitlist Control on Joint Pain Related to Aromatase Inhibitors Among Women With Early-Stage Breast Cancer: A Randomized Clinical Trial.JAMA. 2018 Jul 10;320(2):167-176. doi: 10.1001/jama.2018.8907. JAMA. 2018. PMID: 29998338 Free PMC article. Clinical Trial.
-
Electroacupuncture for fatigue, sleep, and psychological distress in breast cancer patients with aromatase inhibitor-related arthralgia: a randomized trial.Cancer. 2014 Dec 1;120(23):3744-51. doi: 10.1002/cncr.28917. Epub 2014 Jul 30. Cancer. 2014. PMID: 25077452 Free PMC article. Clinical Trial.
-
Feasibility trial of electroacupuncture for aromatase inhibitor--related arthralgia in breast cancer survivors.Integr Cancer Ther. 2009 Jun;8(2):123-9. doi: 10.1177/1534735409332903. Integr Cancer Ther. 2009. PMID: 19679620 Free PMC article. Clinical Trial.
-
Effect of acupuncture on aromatase inhibitor-induced arthralgia in patients with breast cancer: A meta-analysis of randomized controlled trials.Breast. 2017 Jun;33:132-138. doi: 10.1016/j.breast.2017.03.015. Epub 2017 Apr 4. Breast. 2017. PMID: 28384564 Review.
-
Acupuncture for Arthralgia Induced by Aromatase Inhibitors in Patients with Breast Cancer: A Systematic Review and Meta-analysis.Integr Cancer Ther. 2021 Jan-Dec;20:1534735420980811. doi: 10.1177/1534735420980811. Integr Cancer Ther. 2021. PMID: 33586504 Free PMC article.
Cited by
-
Effect of Acupuncture vs Sham Acupuncture or Waitlist Control on Joint Pain Related to Aromatase Inhibitors Among Women With Early-Stage Breast Cancer: A Randomized Clinical Trial.JAMA. 2018 Jul 10;320(2):167-176. doi: 10.1001/jama.2018.8907. JAMA. 2018. PMID: 29998338 Free PMC article. Clinical Trial.
-
Acupuncture at KI3 in healthy volunteers induces specific cortical functional activity: an fMRI study.BMC Complement Altern Med. 2015 Oct 14;15:361. doi: 10.1186/s12906-015-0881-3. BMC Complement Altern Med. 2015. PMID: 26467429 Free PMC article. Clinical Trial.
-
Acupuncture for Aromatase Inhibitor-Induced Arthralgia in Breast Cancer: An Umbrella Review.Breast Care (Basel). 2024 Oct;19(5):252-269. doi: 10.1159/000540749. Epub 2024 Aug 8. Breast Care (Basel). 2024. PMID: 39439861 Review.
-
Comparison of Acupuncture vs Sham Acupuncture or Waiting List Control in the Treatment of Aromatase Inhibitor-Related Joint Pain: A Randomized Clinical Trial.JAMA Netw Open. 2022 Nov 1;5(11):e2241720. doi: 10.1001/jamanetworkopen.2022.41720. JAMA Netw Open. 2022. PMID: 36367721 Free PMC article. Clinical Trial.
-
Integrative Medicine Therapies for Pain Management in Cancer Patients.Cancer J. 2019 Sep/Oct;25(5):343-348. doi: 10.1097/PPO.0000000000000399. Cancer J. 2019. PMID: 31567462 Free PMC article. Review.
References
-
- Burstein HJ, Winer EP. Aromatase inhibitors and arthralgias: a new frontier in symptom management for breast cancer survivors. J Clin Oncol. 2007 Sep 1;25(25):3797–3799. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

