Patient-reported outcomes in randomised controlled trials of prostate cancer: methodological quality and impact on clinical decision making
- PMID: 24210091
- PMCID: PMC4150854
- DOI: 10.1016/j.eururo.2013.10.017
Patient-reported outcomes in randomised controlled trials of prostate cancer: methodological quality and impact on clinical decision making
Abstract
Context: Patient-reported outcomes (PRO) data from randomised controlled trials (RCTs) are increasingly used to inform patient-centred care as well as clinical and health policy decisions.
Objective: The main objective of this study was to investigate the methodological quality of PRO assessment in RCTs of prostate cancer (PCa) and to estimate the likely impact of these studies on clinical decision making.
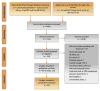
Evidence acquisition: A systematic literature search of studies was undertaken on main electronic databases to retrieve articles published between January 2004 and March 2012. RCTs were evaluated on a predetermined extraction form, including (1) basic trial demographics and clinical and PRO characteristics; (2) level of PRO reporting based on the recently published recommendations by the International Society for Quality of Life Research; and (3) bias, assessed using the Cochrane Risk of Bias tool. Studies were systematically analysed to evaluate their relevance for supporting clinical decision making.
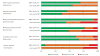
Evidence synthesis: Sixty-five RCTs enrolling a total of 22 071 patients were evaluated, with 31 (48%) in patients with nonmetastatic disease. When a PRO difference between treatments was found, it related in most cases to symptoms only (n=29, 58%). Although the extent of missing data was generally documented (72% of RCTs), few reported details on statistical handling of this data (18%) and reasons for dropout (35%). Improvements in key methodological aspects over time were found. Thirteen (20%) RCTs were judged as likely to be robust in informing clinical decision making. Higher-quality PRO studies were generally associated with those RCTs that had higher internal validity.
Conclusions: Including PRO in RCTs of PCa patients is critical for better evaluating the treatment effectiveness of new therapeutic approaches. Marked improvements in PRO quality reporting over time were found, and it is estimated that at least one-fifth of PRO RCTs have provided sufficient details to allow health policy makers and physicians to make critical appraisals of results.
Patient summary: In this report, we have investigated the methodological quality of PCa trials that have included a PRO assessment. We conclude that including PRO is critical to better evaluating the treatment effectiveness of new therapeutic approaches from the patient's perspective. Also, at least one-fifth of PRO RCTs in PCa have provided sufficient details to allow health policy makers and physicians to make a critical appraisal of results.
Keywords: Clinical decision making; Clinical trials; Patient-reported outcomes; Prostate cancer; Quality of life.
Copyright © 2013. Published by Elsevier B.V.
Figures

Comment in
-
Times, they are a-changing.Eur Urol. 2014 Sep;66(3):428-9. doi: 10.1016/j.eururo.2013.11.043. Epub 2013 Dec 8. Eur Urol. 2014. PMID: 24332346 No abstract available.
References
-
- Boyle P, Levin B, editors. World Health Organization. World Cancer Report. Lyon, France: IARC Press; 2008.
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. - PubMed
-
- Wolf AM, Wender RC, Etzioni RB, et al. American Cancer Society Prostate Cancer Advisory Committee. American Cancer Society guideline for the early detection of prostate cancer: update 2010. CA Cancer J Clin. 2010;60:70–98. - PubMed
-
- Singh J, Trabulsi EJ, Gomella LG. The quality-of-life impact of prostate cancer treatments. Curr Urol Rep. 2010;11:139–46. - PubMed
-
- US Food and Drug Administration. Guidance for industry. Patient-reported outcome measures: use in medical product development to support labeling claims. US Department of Health and Human Services Food and Drug Administration Web site. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformati.... Updated December 2009. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

