Regulation of hematopoietic stem cells by bone marrow stromal cells
- PMID: 24210164
- PMCID: PMC3947365
- DOI: 10.1016/j.it.2013.10.002
Regulation of hematopoietic stem cells by bone marrow stromal cells
Abstract
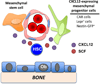
Hematopoietic stem cells (HSCs) reside in specialized microenvironments (niches) in the bone marrow. The stem cell niche is thought to provide signals that support key HSC properties, including self-renewal capacity and long-term multilineage repopulation ability. The stromal cells that comprise the stem cell niche and the signals that they generate that support HSC function are the subjects of intense investigation. Here, we review the complex and diverse stromal cell populations that reside in the bone marrow and examine their contribution to HSC maintenance. We highlight recent data suggesting that perivascular chemokine CXC ligand (CXCL)12-expressing mesenchymal progenitors and endothelial cells are key cellular components of the stem cell niche in the bone marrow.
Keywords: CXCL12; hematopoietic stem cells; mesenchymal stem cells; osteoblasts; stem cell niche.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Disclosures: The authors have no conflicts of interest to disclose
Figures


References
-
- Kiel MJ, et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. - PubMed
-
- Osawa M, et al. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science. 1996;273:242–245. - PubMed
-
- Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

