Self-assembly of VPS41 promotes sorting required for biogenesis of the regulated secretory pathway
- PMID: 24210660
- PMCID: PMC3974617
- DOI: 10.1016/j.devcel.2013.10.007
Self-assembly of VPS41 promotes sorting required for biogenesis of the regulated secretory pathway
Abstract
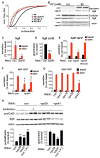
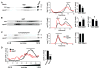
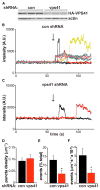
The regulated release of polypeptides has a central role in physiology, behavior, and development, but the mechanisms responsible for production of the large dense core vesicles (LDCVs) capable of regulated release have remained poorly understood. Recent work has implicated cytosolic adaptor protein AP-3 in the recruitment of LDCV membrane proteins that confer regulated release. However, AP-3 in mammals has been considered to function in the endolysosomal pathway and in the biosynthetic pathway only in yeast. We now find that the mammalian homolog of yeast VPS41, a member of the homotypic fusion and vacuole protein sorting (HOPS) complex that delivers biosynthetic cargo to the endocytic pathway in yeast, promotes LDCV formation through a common mechanism with AP-3, indicating a conserved role for these proteins in the biosynthetic pathway. VPS41 also self-assembles into a lattice, suggesting that it acts as a coat protein for AP-3 in formation of the regulated secretory pathway.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures




References
-
- Aoyama M, Sun-Wada GH, Yamamoto A, Yamamoto M, Hamada H, Wada Y. Spatial restriction of bone morphogenetic protein signaling in mouse gastrula through the mVam2-dependent endocytic pathway. Dev Cell. 2012;22:1163–1175. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

