High-resolution replication profiles define the stochastic nature of genome replication initiation and termination
- PMID: 24210825
- PMCID: PMC3898788
- DOI: 10.1016/j.celrep.2013.10.014
High-resolution replication profiles define the stochastic nature of genome replication initiation and termination
Abstract
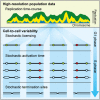
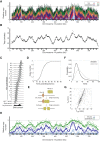
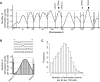
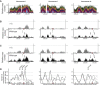
Eukaryotic genome replication is stochastic, and each cell uses a different cohort of replication origins. We demonstrate that interpreting high-resolution Saccharomyces cerevisiae genome replication data with a mathematical model allows quantification of the stochastic nature of genome replication, including the efficiency of each origin and the distribution of termination events. Single-cell measurements support the inferred values for stochastic origin activation time. A strain, in which three origins were inactivated, confirmed that the distribution of termination events is primarily dictated by the stochastic activation time of origins. Cell-to-cell variability in origin activity ensures that termination events are widely distributed across virtually the whole genome. We propose that the heterogeneity in origin usage contributes to genome stability by limiting potentially deleterious events from accumulating at particular loci.
Copyright © 2013 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Agier N., Fischer G. The mutational profile of the yeast genome is shaped by replication. Mol. Biol. Evol. 2012;29:905–913. - PubMed
Publication types
MeSH terms
Associated data
- Actions
Grants and funding
- BB/ G001596/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- 080737/WT_/Wellcome Trust/United Kingdom
- BB/E023754/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- 097945/WT_/Wellcome Trust/United Kingdom
- BB/G001596/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

