Induction and stability of the anergic phenotype in T cells
- PMID: 24211041
- PMCID: PMC3927995
- DOI: 10.1016/j.smim.2013.10.010
Induction and stability of the anergic phenotype in T cells
Abstract
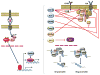
One of the mechanisms that are in place to control the activation of mature T cells that bear self-reactive antigen receptors is anergy, a long-term state of hyporesponsiveness that is established in T cells in response to suboptimal stimulation. T cells receive signals that result not only from antigen recognition and costimulation but also from other sources, including cytokine receptors, inhibitory receptors or metabolic sensors. Integration of those signals will determine T cell fate. Under conditions that induce anergy, T cells activate a program of gene expression that leads to the production of proteins that block T cell receptor signaling and inhibit cytokine gene expression. In this review we will examine those signals that determine functional outcome following antigen encounter, review current knowledge of the factors that ensure signaling inhibition and epigenetic gene silencing in anergic cells and explore the mechanisms that lead to the reversal of anergy and the reacquisition of effector functions.
Keywords: Anergy induction; Anergy reversal; Epigenetics; NFAT.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures
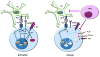
Similar articles
-
Early growth response gene-2, a zinc-finger transcription factor, is required for full induction of clonal anergy in CD4+ T cells.J Immunol. 2004 Dec 15;173(12):7331-8. doi: 10.4049/jimmunol.173.12.7331. J Immunol. 2004. PMID: 15585857
-
Induction of T cell anergy: integration of environmental cues and infectious tolerance.Curr Opin Immunol. 2010 Oct;22(5):552-9. doi: 10.1016/j.coi.2010.08.005. Epub 2010 Sep 24. Curr Opin Immunol. 2010. PMID: 20869863 Free PMC article. Review.
-
Threshold signaling of human Th0 cells in activation and anergy: modulation of effector function by altered TCR ligand.J Immunol. 2000 Jun 1;164(11):6034-40. doi: 10.4049/jimmunol.164.11.6034. J Immunol. 2000. PMID: 10820288
-
Genetic and biochemical regulation of CD4 T cell effector differentiation: insights from examination of T cell clonal anergy.Immunol Res. 2010 Jul;47(1-3):162-71. doi: 10.1007/s12026-009-8147-0. Immunol Res. 2010. PMID: 20077160 Free PMC article. Review.
-
Human T cell activation by costimulatory signal-deficient allogeneic cells induces inducible costimulator-expressing anergic T cells with regulatory cell activity.J Immunol. 2004 May 1;172(9):5371-8. doi: 10.4049/jimmunol.172.9.5371. J Immunol. 2004. PMID: 15100277
Cited by
-
NFAT-dependent and -independent exhaustion circuits program maternal CD8 T cell hypofunction in pregnancy.J Exp Med. 2022 Jan 3;219(1):e20201599. doi: 10.1084/jem.20201599. Epub 2021 Dec 9. J Exp Med. 2022. PMID: 34882194 Free PMC article.
-
The Role of Microbiota-Derived Vitamins in Immune Homeostasis and Enhancing Cancer Immunotherapy.Cancers (Basel). 2023 Feb 18;15(4):1300. doi: 10.3390/cancers15041300. Cancers (Basel). 2023. PMID: 36831641 Free PMC article. Review.
-
Diacylglycerol Kinases and Its Role in Lipid Metabolism and Related Diseases.Int J Mol Sci. 2024 Dec 9;25(23):13207. doi: 10.3390/ijms252313207. Int J Mol Sci. 2024. PMID: 39684917 Free PMC article. Review.
-
Functions of the FAK family kinases in T cells: beyond actin cytoskeletal rearrangement.Immunol Res. 2014 Aug;59(1-3):23-34. doi: 10.1007/s12026-014-8527-y. Immunol Res. 2014. PMID: 24816556 Free PMC article. Review.
-
Refractory T-Cell Anergy and Rapidly Fatal Progressive Multifocal Leukoencephalopathy After Prolonged CTLA4 Therapy.Open Forum Infect Dis. 2017 May 16;4(2):ofx100. doi: 10.1093/ofid/ofx100. eCollection 2017 Spring. Open Forum Infect Dis. 2017. PMID: 28638849 Free PMC article.
References
-
- Palmer E. Negative selection--clearing out the bad apples from the T-cell repertoire. Nat Rev Immunol. 2003;3:383–391. - PubMed
-
- Hogquist KA, Baldwin TA, Jameson SC. Central tolerance: learning self-control in the thymus. Nat Rev Immunol. 2005;5:772–782. - PubMed
-
- Wells AD. New insights into the molecular basis of T cell anergy: anergy factors, avoidance sensors, and epigenetic imprinting. J Immunol. 2009;182:7331–7341. - PubMed
-
- Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol. 2010;11:7–13. - PubMed
-
- Mueller DL. Mechanisms maintaining peripheral tolerance. Nat Immunol. 2010;11:21–27. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

