Arsenic trioxide induces apoptosis in B-cell chronic lymphocytic leukemic cells through down-regulation of survivin via the p53-dependent signaling pathway
- PMID: 24211095
- PMCID: PMC5310943
- DOI: 10.1016/j.leukres.2013.09.019
Arsenic trioxide induces apoptosis in B-cell chronic lymphocytic leukemic cells through down-regulation of survivin via the p53-dependent signaling pathway
Abstract
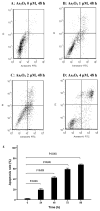
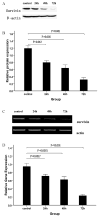
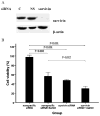
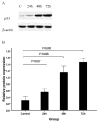
Arsenic trioxide (As2O3) can induce apoptosis in many tumors. However, the associated mechanisms are not clearly understood. We found that As2O3 significantly inhibited the proliferation of WSU-CLL cells and induced apoptosis in dose- and time-dependent manners. WSU-CLL cells treated with 2μM As2O3 showed survivin down-regulation and p53 up-regulation. Survivin siRNA combined with As2O3 further inhibited the proliferation of WSU-CLL cells. p53 inhibition by siRNA prevented the down-regulation of survivin by As2O3 and prevented the As2O3-induced cytotoxicity of WSU-CLL cells. These results suggest that As2O3 may be of therapeutic value for chronic lymphocytic leukemia.
Keywords: Apoptosis; Arsenic trioxide; P53 and CLL; Survivin; WSU-CLL.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Conflict of interest statement
All authors declare no conflict of interest.
Figures



Comment in
-
Cell line cross-contamination: WSU-CLL is a known derivative of REH and is unsuitable as a model for chronic lymphocytic leukaemia.Leuk Res. 2014 Aug;38(8):999-1001. doi: 10.1016/j.leukres.2014.05.003. Epub 2014 May 23. Leuk Res. 2014. PMID: 24923861 No abstract available.
References
-
- Caligaris-Cappio F, Hamblin TJ. B-cell chronic lymphocytic leukemia: a bird of a different feather. J Clin Oncol. 1999;17:399–408. - PubMed
-
- Li HM, Long Y, Qing C, Yu M, Li ZH, Zhang XM, et al. Arsenic trioxide induces apoptosis of Burkitt lymphoma cell lines through multiple apoptotic pathways and triggers antiangiogenesis. Oncol Res. 2011;19:149–63. - PubMed
-
- Pettersson HM, Pietras A, Munksgaard Persson M, Karlsson J, Johansson L, Shoshan MC, et al. Arsenic trioxide is highly cytotoxic to small cell lung carcinoma cells. Mol Cancer Ther. 2009;8:160–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

