Docetaxel and dasatinib or placebo in men with metastatic castration-resistant prostate cancer (READY): a randomised, double-blind phase 3 trial
- PMID: 24211163
- PMCID: PMC5478530
- DOI: 10.1016/S1470-2045(13)70479-0
Docetaxel and dasatinib or placebo in men with metastatic castration-resistant prostate cancer (READY): a randomised, double-blind phase 3 trial
Abstract
Background: Src kinase-mediated interactions between prostate cancer cells and osteoclasts might promote bone metastasis. Dasatinib inhibits tyrosine kinases, including Src kinases. Data suggests that dasatinib kinase inhibition leads to antitumour activity, affects osteoclasts, and has synergy with docetaxel, a first-line chemotherapy for metastatic castration-resistant prostate cancer. We assessed whether dasatinib plus docetaxel in chemotherapy-naive men with metastatic castration-resistant prostate cancer led to greater efficacy than with docetaxel alone.
Methods: In this double-blind, randomised, placebo-controlled phase 3 study, we enrolled men of 18 years or older with chemotherapy-naive, metastatic, castration-resistant prostate cancer, and adequate organ function from 186 centres across 25 countries. Eligible patients were randomly assigned (1:1) via an interactive voice response system to receive docetaxel (75 mg/m(2) intravenously every 3 weeks, plus oral prednisone 5 mg twice daily), plus either dasatinib (100 mg orally once daily) or placebo until disease progression or unacceptable toxicity. Randomisation was stratified by Eastern Cooperative Oncology Group performance status (0-1 vs 2), bisphosphonate use (yes vs no), and urinary N-telopeptide (uNTx) value (<60 μmol/mol creatinine vs ≥60 μmol/mol creatinine). All patients, investigators, and personnel involved in study conduct and data analyses were blinded to treatment allocation. The primary endpoint was overall survival, analysed by intention to treat. The trial is registered with ClinicalTrials.gov, number NCT00744497.
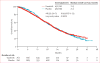
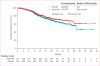
Findings: Between Oct 30, 2008, and April 11, 2011, 1522 eligible patients were randomly assigned to treatment; 762 patients were assigned to dasatinib and 760 to placebo. At final analysis, median follow-up was 19·0 months (IQR 11·2-25·1) and 914 patients had died. Median overall survival was 21·5 months (95% CI 20·3-22·8) in the dasatinib group and 21·2 months (20·0-23·4) in the placebo group (stratified hazard ratio [HR] 0·99, 95·5% CI 0·87-1·13; p=0·90). The most common grade 3-4 adverse events included diarrhoea (58 [8%] patients in the dasatinib group vs 27 [4%] patients in the placebo group), fatigue (62 [8%] vs 42 [6%]), and asthenia (40 [5%] vs 23 [3%]); grade 3-4 pleural effusions were uncommon (ten [1%] vs three [<1%]).
Interpretation: The addition of dasatinib to docetaxel did not improve overall survival for chemotherapy-naive men with metastatic castration-resistant prostate cancer. This study does not support the combination of dasatinib and docetaxel in this population of patients.
Funding: Bristol-Myers Squibb.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Conflict of interest statement
GCT, PP, SD, and SC are full-time employees of Bristol-Myers Squibb, and own company stocks and stock options. AJA has served as a consultant for Sanofi-Aventis, and has received research funding from Bristol-Myers Squibb and Sanofi-Aventis. FS has received research funding from Bristol-Myers Squibb. GW has received honoraria from Bristol-Myers Squibb. EE has received honoraria from Johnson & Johnson, Sanofi-Aventis, and Takeda; has participated on the speakers’ bureau for Johnson & Johnson; and has received research funding from Sanofi-Aventis and Astellas/Medivation. SO has received honoraria from Bristol-Myers Squibb. MJM has served as a consultant for Bayer, Janssen, and Takeda and has received research funding from Algeta, Bayer, Sanofi-Aventis, and Takeda. BS has received honoraria and travel support from Bristol-Myers Squibb. All other authors declare that they have no conflicts of interest.
Figures

Comment in
-
Dasatinib and docetaxel in advanced prostate cancer.Lancet Oncol. 2013 Dec;14(13):1248-9. doi: 10.1016/S1470-2045(13)70500-X. Epub 2013 Nov 8. Lancet Oncol. 2013. PMID: 24211164 No abstract available.
-
Prostate cancer: Dasatinib fails to improve on docetaxel for metastatic CRPC.Nat Rev Urol. 2014 Jan;11(1):5. doi: 10.1038/nrurol.2013.283. Epub 2013 Dec 3. Nat Rev Urol. 2014. PMID: 24296705 No abstract available.
-
Pharmacokinetic interactions of dasatinib and docetaxel.Lancet Oncol. 2014 Feb;15(2):e51. doi: 10.1016/S1470-2045(14)70004-X. Lancet Oncol. 2014. PMID: 24480555 No abstract available.
References
-
- Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–20. - PubMed
-
- Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12. - PubMed
-
- de Bono JS, Oudard S, Ozguroglu M, et al. for the TROPIC Investigators Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–54. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

