Reduction in HPV 16/18 prevalence in sexually active young women following the introduction of HPV immunisation in England
- PMID: 24211166
- PMCID: PMC3898718
- DOI: 10.1016/j.vaccine.2013.10.085
Reduction in HPV 16/18 prevalence in sexually active young women following the introduction of HPV immunisation in England
Abstract
Background: Reduction in the prevalence of vaccine type HPV infection in young women is an early indication of the impact of the HPV immunisation programme and a necessary outcome if the subsequent impact on cervical cancer is to be realised.
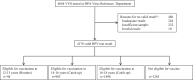
Methods: Residual vulva-vaginal swab (VVS) specimens from young women aged 16-24 years undergoing chlamydia screening in community sexual health services (formerly known as family planning clinics), general practice (GP), and youth clinics in 2010-2012 were submitted from 10 laboratories in seven regions around England. These specimens were linked to demographic and sexual behaviour data reported with the chlamydia test, anonymised, and tested for type-specific HPV DNA using a multiplex PCR and Luminex-based genotyping test. Estimated immunisation coverage was calculated and findings were compared to a baseline survey conducted prior to the introduction of HPV immunisation in 2008.
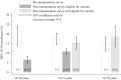
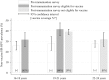
Results: A total of 4664 eligible specimens were collected and 4178 had a valid test result. The post-immunisation prevalence of HPV 16/18 infection was lowest in this youngest age group (16-18 years) and increased with age. This increase with age was a reversal of the pattern seen prior to immunisation and was inversely associated with estimates of age-specific immunisation coverage (65% for 16-18 year olds). The prevalence of HPV 16/18 infection in the post-immunisation survey was 6.5% amongst 16-18 year olds, compared to 19.1% in the similar survey conducted prior to the introduction of HPV immunisation.
Conclusions: These findings are the first indication that the national HPV immunisation programme is successfully preventing HPV 16/18 infection in sexually active young women in England. The reductions seen suggest, for the estimated coverage, high vaccine effectiveness and some herd-protection benefits. Continued surveillance is needed to determine the effects of immunisation on non-vaccine HPV types.
Keywords: CI; Cervarix; Confidence Interval; GP; General Practice; HC2; HPV; HPV immunisation; HPV prevalence; HR; High-risk; Human Papillomavirus; Hybrid Capture 2; LA; LR; LSOA; Linear Array; Low-risk; Lower Super Output Area; NCSP; National Chlamydia Screening Programme; OR; Odds ratio; PCR; PCT; PDH; PHE; Polymerase Chain Reaction; Primary Care Trust; Public Health England; Pyruvate dehydrogenase; REC; Research Ethics Committee; Surveillance; VVS; Vulva-vaginal Swab.
Crown Copyright © 2013. Published by Elsevier Ltd. All rights reserved.
Figures
References
-
- De Carvalho N., Teixeira J., Roteli-Martins C.M. Sustained efficacy and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine up to 7.3 years in young adult women. Vaccine. 2010;28:6247–6255. - PubMed
-
- Lehtinen M., Paavonen J., Wheeler C.M. Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2011 - PubMed
-
- World Health Organization, Number of countries having introduced HPV vaccines to date, Available from http://www.who.int/nuvi/hpv/decision_implementation/en/index.html (accessed September 2012).
-
- Department of Health, Annual HPV vaccine uptake in England: 2010/11, Available from URL: https://www.wp.dh.gov.uk/immunisation/files/2012/03/120319_HPV_UptakeRep... (accessed October 2012).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

