Interleukin-1β enhances neuronal vulnerability to proNGF-mediated apoptosis by increasing surface expression of p75(NTR) and sortillin
- PMID: 24211304
- PMCID: PMC3947643
- DOI: 10.1016/j.neuroscience.2013.10.058
Interleukin-1β enhances neuronal vulnerability to proNGF-mediated apoptosis by increasing surface expression of p75(NTR) and sortillin
Abstract
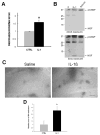
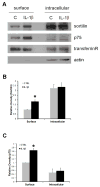
Many types of injury such as seizure, ischemia, and oxidative stress cause upregulation of the p75 neurotrophin receptor (p75(NTR)) in brain neurons, where it promotes apoptosis, however the mechanism by which p75(NTR) is regulated under these conditions is not well understood. Proinflammatory cytokines such as interleukin-1β (IL-1β) are highly produced under these injury conditions and, in particular, are expressed rapidly in the rat hippocampus after seizure. IL-1β is known to increase neuronal vulnerability under many conditions, although it does not directly induce neuronal death. Recently, we have shown that these cytokines regulate p75(NTR) induction both in neurons and astrocytes in vitro. Here, we show that IL-1β infusion into the brain induces p75(NTR) in neurons of the CA1 area of the hippocampus. While IL-1β induction of p75(NTR) is not sufficient to induce cell death, we demonstrate that IL-1β primes the neurons by recruiting p75(NTR) and its coreceptor sortilin to the cell surface, making the neurons more vulnerable to subsequent challenge by proNGF. These results suggest a mechanism by which IL-1β exacerbates neuronal death following injury.
Keywords: CSF; EDTA; IL-1β; NGF; TdT; apoptosis; brain injury; cerebrospinal fluid; ethylenediaminetetraacetic acid; interleukin-1; interleukin-1β; neurotrophin; p75; p75 neurotrophin receptor; p75(NTR); terminal deoxynucleotidyl transferase.
Copyright © 2013 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures



Similar articles
-
Proneurotrophin-3 may induce Sortilin-dependent death in inner ear neurons.Eur J Neurosci. 2011 Feb;33(4):622-31. doi: 10.1111/j.1460-9568.2010.07556.x. Epub 2011 Jan 24. Eur J Neurosci. 2011. PMID: 21261755 Free PMC article.
-
Induction of proneurotrophins and activation of p75NTR-mediated apoptosis via neurotrophin receptor-interacting factor in hippocampal neurons after seizures.J Neurosci. 2008 Sep 24;28(39):9870-9. doi: 10.1523/JNEUROSCI.2841-08.2008. J Neurosci. 2008. PMID: 18815271 Free PMC article.
-
p75 Neurotrophin receptor signaling regulates hepatic myofibroblast proliferation and apoptosis in recovery from rodent liver fibrosis.Hepatology. 2009 Mar;49(3):901-10. doi: 10.1002/hep.22701. Hepatology. 2009. PMID: 19072833
-
Proteolytic processing of the p75 neurotrophin receptor: A prerequisite for signalling?: Neuronal life, growth and death signalling are crucially regulated by intra-membrane proteolysis and trafficking of p75(NTR).Bioessays. 2011 Aug;33(8):614-25. doi: 10.1002/bies.201100036. Epub 2011 Jun 30. Bioessays. 2011. PMID: 21717487 Review.
-
Structural Characterization of the p75 Neurotrophin Receptor: A Stranger in the TNFR Superfamily.Vitam Horm. 2017;104:57-87. doi: 10.1016/bs.vh.2016.10.007. Epub 2016 Nov 29. Vitam Horm. 2017. PMID: 28215307 Review.
Cited by
-
Chemokine CCL2-CCR2 Signaling Induces Neuronal Cell Death via STAT3 Activation and IL-1β Production after Status Epilepticus.J Neurosci. 2017 Aug 16;37(33):7878-7892. doi: 10.1523/JNEUROSCI.0315-17.2017. Epub 2017 Jul 17. J Neurosci. 2017. PMID: 28716963 Free PMC article.
-
Sortilin is dispensable for secondary injury processes following traumatic brain injury in mice.Heliyon. 2024 Jul 29;10(15):e35198. doi: 10.1016/j.heliyon.2024.e35198. eCollection 2024 Aug 15. Heliyon. 2024. PMID: 39170542 Free PMC article.
-
A novel antagonist of p75NTR reduces peripheral expansion and CNS trafficking of pro-inflammatory monocytes and spares function after traumatic brain injury.J Neuroinflammation. 2016 Apr 22;13(1):88. doi: 10.1186/s12974-016-0544-4. J Neuroinflammation. 2016. PMID: 27102880 Free PMC article.
-
Etiological Roles of p75NTR in a Mouse Model of Wet Age-Related Macular Degeneration.Cells. 2023 Jan 12;12(2):297. doi: 10.3390/cells12020297. Cells. 2023. PMID: 36672232 Free PMC article.
-
Inflammatory cytokines TNFα, IL-1β, and IL-6 are induced in endotoxin- stimulated microglia through different signaling cascades.Sci Prog. 2021 Oct;104(4):368504211054985. doi: 10.1177/00368504211054985. Sci Prog. 2021. PMID: 34821182 Free PMC article.
References
-
- Allan SM, Tyrrell PJ, Rothwell NJ. Interleukin-1 and neuronal injury. Nat Rev Immunol. 2005;5:629–640. - PubMed
-
- Armstrong DM, Brady R, Hersh LB, Hayes RC, Wiley RG. Expression of choline acetyltransferase and nerve growth factor receptor within hypoglossal motoneurons following nerve injury. The Journal of comparative neurology. 1991;304:596–607. - PubMed
-
- Casha S, Yu WR, Fehlings MG. Oligodendroglial apoptosis occurs along degenerating axons and is associated with FAS and p75 expression following spinal cord injury in the rat. Neuroscience. 2001;103:203–218. - PubMed
-
- Chao CC, Hu S, Ehrlich L, Peterson PK. Interleukin-1 and tumor necrosis factor-alpha synergistically mediate neurotoxicity: involvement of nitric oxide and of N-methyl-D-aspartate receptors. Brain Behav Immun. 1995;9:355–365. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

